Increased Awareness and Education
The heightened awareness and education regarding respiratory health are driving the Acute Respiratory Distress Syndrome Market. Public health campaigns and educational initiatives are informing individuals about the risks and symptoms associated with acute respiratory distress syndrome. This increased awareness is likely to lead to earlier diagnosis and treatment, ultimately improving patient outcomes. Healthcare professionals are also receiving enhanced training on recognizing and managing respiratory distress, which is crucial for effective intervention. As more individuals seek medical attention for respiratory issues, the demand for specialized treatments and therapies is expected to grow. This trend underscores the importance of continuous education and awareness in addressing the challenges posed by acute respiratory distress syndrome.
Emergence of Personalized Medicine
The rise of personalized medicine is influencing the Acute Respiratory Distress Syndrome Market significantly. Tailored treatment approaches based on individual patient profiles are becoming more prevalent, allowing for more effective management of acute respiratory distress syndrome. Advances in genomics and biomarker research are facilitating the development of targeted therapies that address the specific needs of patients. This shift towards personalized care is likely to enhance treatment efficacy and reduce adverse effects, thereby improving overall patient satisfaction. As healthcare providers increasingly adopt personalized medicine strategies, the market for acute respiratory distress syndrome treatments is expected to expand. The potential for individualized therapies to revolutionize patient care in respiratory conditions is a promising development for the industry.
Rising Incidence of Respiratory Diseases
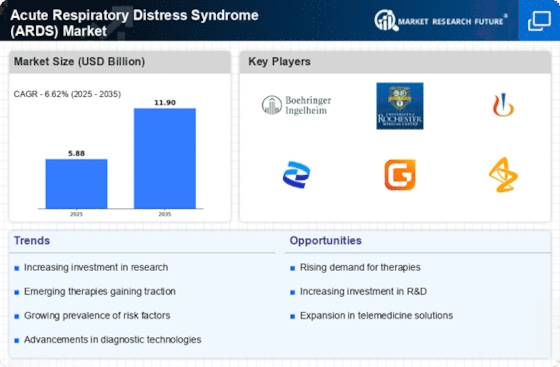
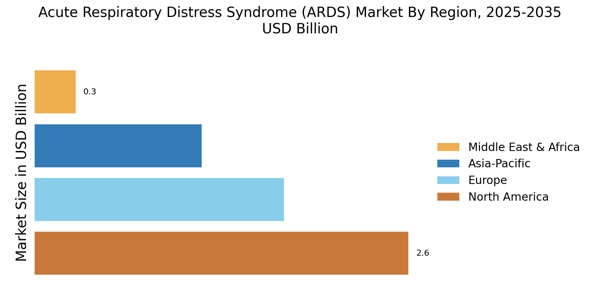
The increasing prevalence of respiratory diseases is a primary driver for the Acute Respiratory Distress Syndrome Market. Factors such as urbanization, pollution, and lifestyle changes contribute to a higher incidence of conditions that can lead to acute respiratory distress. According to recent data, respiratory diseases account for a significant portion of global morbidity and mortality, necessitating effective treatment options. This trend is likely to propel the demand for advanced therapies and medical interventions aimed at managing acute respiratory distress syndrome. As healthcare systems adapt to these challenges, investments in research and development are expected to rise, further stimulating the market. The growing awareness of respiratory health issues among the population also plays a crucial role in driving the market forward.
Technological Advancements in Medical Devices
Technological innovations in medical devices are transforming the Acute Respiratory Distress Syndrome Market. The development of advanced ventilators, monitoring systems, and therapeutic equipment enhances patient care and outcomes. For instance, the introduction of non-invasive ventilation techniques has shown promising results in managing acute respiratory distress syndrome, leading to reduced mortality rates. Furthermore, the integration of artificial intelligence and machine learning in diagnostic tools is improving the accuracy of early detection and treatment. As healthcare providers increasingly adopt these technologies, the market is likely to experience substantial growth. The ongoing research into novel devices and their applications in critical care settings is expected to further bolster the market, addressing the urgent need for effective management of respiratory conditions.
Growing Investment in Healthcare Infrastructure
The expansion of healthcare infrastructure is a significant driver for the Acute Respiratory Distress Syndrome Market. Governments and private entities are investing heavily in healthcare facilities, particularly in regions with high incidences of respiratory diseases. This investment is aimed at enhancing the capacity to treat acute respiratory distress syndrome and related conditions. Recent reports indicate that healthcare spending is projected to increase, leading to the establishment of specialized units and improved access to advanced medical technologies. As healthcare systems evolve, the demand for trained professionals and effective treatment protocols will likely rise, further stimulating the market. The establishment of dedicated respiratory care units is expected to play a pivotal role in managing acute respiratory distress syndrome effectively.