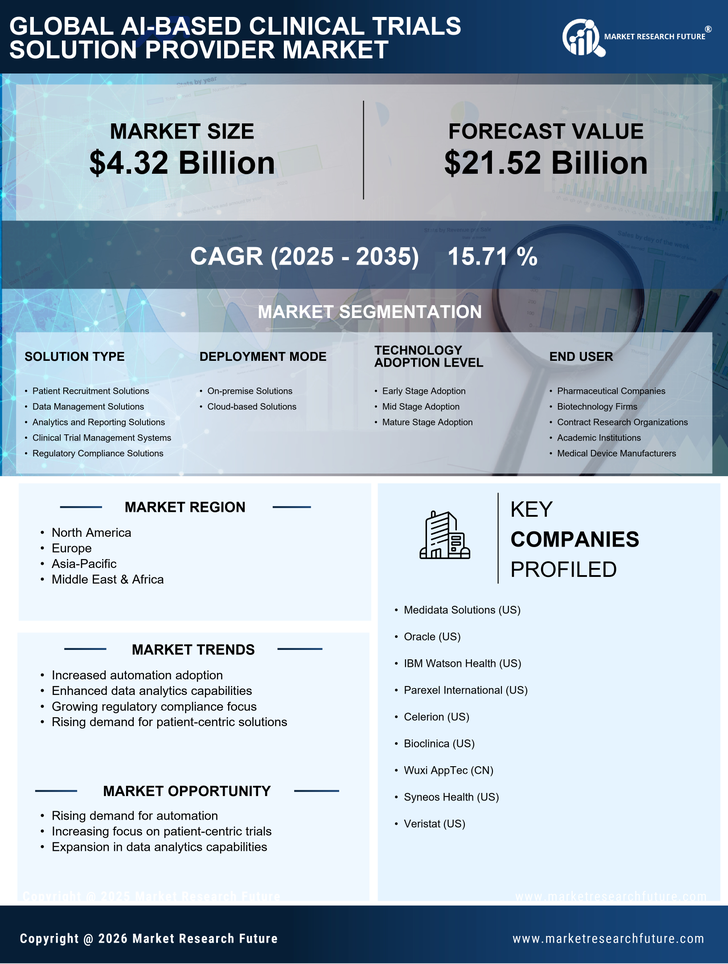
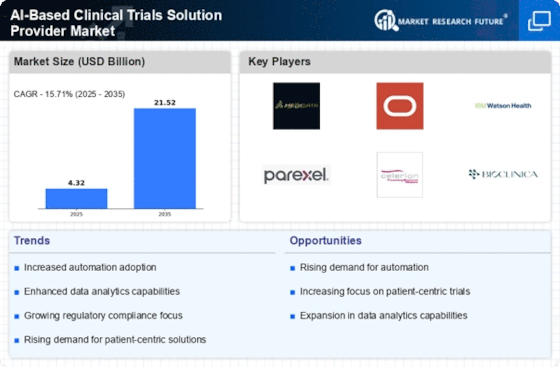
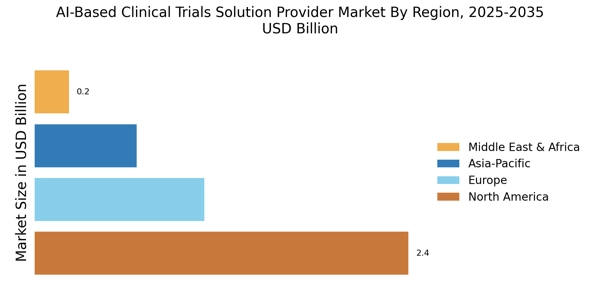
Cost Reduction in Clinical Trials
Cost reduction is a significant driver within the AI-Based Clinical Trials Solution Provider Market. The implementation of AI technologies can lead to substantial savings throughout the clinical trial lifecycle. By automating various processes, such as data collection and analysis, organizations can reduce the time and resources required for trials. Reports suggest that AI can decrease trial costs by up to 30%, which is particularly appealing in an environment where research budgets are often constrained. Furthermore, AI's predictive capabilities can help in identifying potential issues before they arise, allowing for proactive management of resources and timelines. This financial efficiency not only benefits trial sponsors but also enhances the overall sustainability of clinical research, making AI solutions increasingly attractive to stakeholders across the industry.
Enhanced Data Analytics Capabilities
The AI-Based Clinical Trials Solution Provider Market is experiencing a surge in demand for enhanced data analytics capabilities. The integration of artificial intelligence allows for the processing of vast amounts of clinical data, leading to more accurate insights and predictions. This capability is particularly crucial as the industry moves towards more personalized medicine, where treatment efficacy can vary significantly among patient populations. By leveraging AI, clinical trial providers can identify trends and patterns that may not be apparent through traditional analysis methods. This trend is reflected in the increasing investment in AI technologies, with projections indicating that the market for AI in healthcare could reach USD 36 billion by 2025. Such advancements not only improve trial outcomes but also streamline the overall research process, making it more efficient and cost-effective.
Improved Patient Recruitment Strategies
In the AI-Based Clinical Trials Solution Provider Market, improved patient recruitment strategies are becoming a pivotal driver. AI technologies facilitate the identification and engagement of suitable candidates for clinical trials, addressing one of the most significant challenges in the industry. By analyzing electronic health records and other data sources, AI can pinpoint potential participants who meet specific criteria, thereby enhancing recruitment efficiency. This is particularly relevant as studies indicate that nearly 80% of clinical trials fail to meet their enrollment targets. The ability to optimize recruitment not only accelerates trial timelines but also ensures a more diverse participant pool, which is essential for the validity of trial results. As a result, organizations are increasingly adopting AI-driven solutions to enhance their recruitment processes, thereby contributing to the growth of the AI-Based Clinical Trials Solution Provider Market.
Regulatory Compliance and Risk Management
Regulatory compliance and risk management are critical components driving the AI-Based Clinical Trials Solution Provider Market. As regulatory bodies become more receptive to the integration of AI technologies, organizations are leveraging these tools to ensure adherence to stringent guidelines. AI can assist in monitoring compliance in real-time, identifying deviations from protocols, and mitigating risks associated with clinical trials. This capability is particularly vital as the complexity of regulations continues to evolve. Moreover, the ability to analyze historical data and predict potential compliance issues enhances the overall risk management framework. As a result, organizations that adopt AI-driven solutions are likely to experience improved compliance rates, thereby reducing the likelihood of costly delays or penalties. This trend underscores the growing importance of AI in navigating the regulatory landscape of clinical trials.
Advancements in Trial Design and Methodology
Advancements in trial design and methodology are emerging as a key driver in the AI-Based Clinical Trials Solution Provider Market. The application of AI technologies enables researchers to develop more innovative and adaptive trial designs, which can lead to more efficient and effective studies. For instance, AI can facilitate the use of adaptive trial designs that allow for modifications based on interim results, thereby optimizing resource allocation and participant engagement. This flexibility is particularly beneficial in complex therapeutic areas where traditional trial designs may fall short. Furthermore, the ability to simulate various trial scenarios using AI can enhance decision-making processes, leading to better outcomes. As the industry continues to evolve, the integration of AI in trial design is likely to become a standard practice, further propelling the growth of the AI-Based Clinical Trials Solution Provider Market.