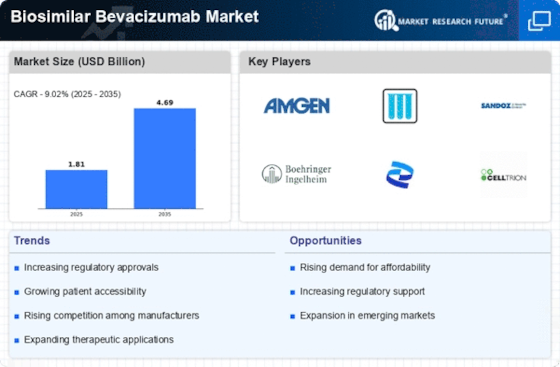
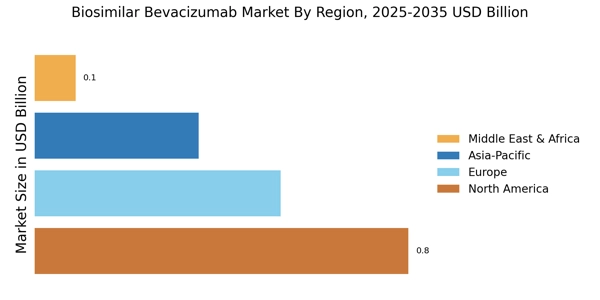
Leading market players are investing heavily in research and development in order to expand their product lines, which will help the biosimilar bevacizumab market grow even more. Market participants are also undertaking a variety of strategic activities to expand their global footprint, with important market developments including new product launches, contractual agreements, mergers and acquisitions, higher investments, and collaboration with other organizations. To expand and survive in a more competitive and rising market climate, the biosimilar bevacizumab industry must offer cost-effective items.
Manufacturing locally to minimize operational costs is one of the key business tactics used by manufacturers in the global biosimilar bevacizumab industry to benefit clients and increase the market sector. In recent years, the biosimilar bevacizumab industry has offered some of the most significant advantages to medicine. Major players in the biosimilar bevacizumab market, including Amgen, AryoGen Pharmed, Biothera, Boehringer Ingelheim, Centus Biotherapeutics, Henlius Biotech, and others, are attempting to increase market demand by investing in research and development operations.
Amgen Inc. is a biotechnology company that discovers, develops, manufactures, and markets innovative human medicines to treat patients suffering from serious diseases. It develops novel medicines in therapeutic areas of cardiovascular, oncology/hematology, inflammation, bone health, neurological disorders, and nephrology. The company develops products using advanced human genetics to analyze the difficulties of disease and understand the fundamentals of human biology. Amgen sells products primarily to pharmaceutical wholesale distributors in the US. It also markets certain products directly to consumers through direct-to-consumer channels and through partnerships with other companies.
The company has a presence in Asia Pacific, Europe, the Middle East, North America, and Australia. Amgen is headquartered in Thousand Oaks, California, the US.
In July Amgen and Allergan announced the launch of Mvasi, a bevacizumab biosimilar referencing Avastin, and Kanjinti, a trastuzumab biosimilar referencing Herceptin, in the United States.
Amneal Pharmaceuticals Inc (Amneal) is a pharmaceutical company that develops, manufactures, and distributes medicines. The company offers generic and specialty pharmaceutical products. Amneal offers products in various dosage forms comprising liquids, sterile injectables, oral solids, powders, nasal sprays, inhalation and respiratory products, transdermal patches, ophthalmics, films, and topicals. It sells its products to wholesalers, chain pharmacies, distributors, hospitals, and individual pharmacies. The company operates in the United States, India, and Ireland. It also operates in distribution centers situated in Pennsylvania, Fountain Run, Kentucky, and Philadelphia. The company has partnered with Baclofen Franchise, Puniska Healthcare Pvt.
Ltd., Kashiv Specialty Pharmaceuticals, LLC, AvKARE, LLC, R&S Northeast LLC, and others. Amneal is headquartered in Bridgewater, New Jersey, the US.
In October Amneal Pharmaceuticals, Inc. announced the commercial launch of ALYMSYS® (bevacizumab-maly), a biosimilar referencing Avastin®. ALYMSYS® is a vascular endothelial growth factor inhibitor used in oncology. This product was developed by mAbxience, a global biotech company with over a decade of experience in the development, manufacture, and commercialization of biopharmaceuticals.