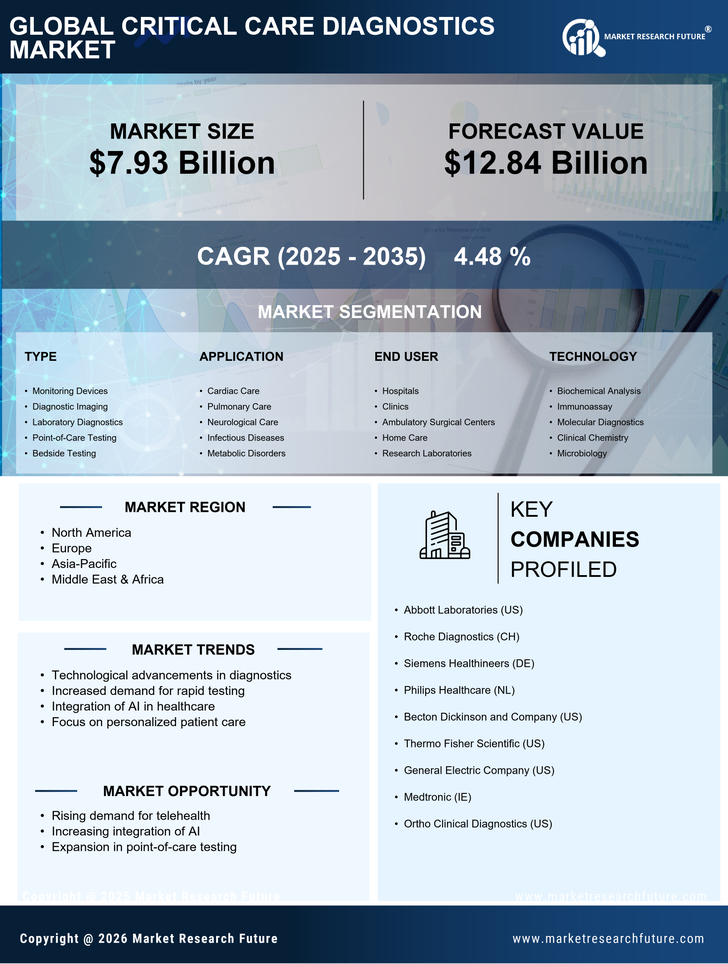
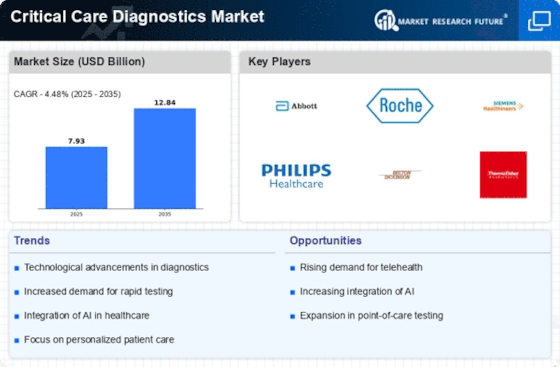
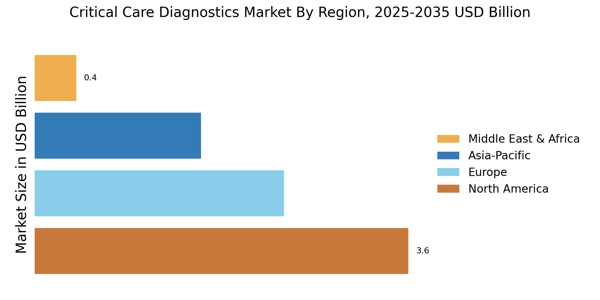
Rising Incidence of Chronic Diseases
The increasing prevalence of chronic diseases such as diabetes, cardiovascular disorders, and respiratory illnesses is a primary driver of the Critical Care Diagnostics Market. As these conditions often require intensive monitoring and management, the demand for advanced diagnostic tools is surging. According to recent data, chronic diseases account for approximately 60% of all deaths worldwide, necessitating effective diagnostic solutions. This trend is likely to propel the market forward, as healthcare providers seek innovative technologies to enhance patient outcomes. The Critical Care Diagnostics Market is thus positioned to expand significantly, driven by the urgent need for timely and accurate diagnostics in critical care settings.
Regulatory Support and Standardization
Regulatory support and standardization are essential drivers of the Critical Care Diagnostics Market. Governments and health organizations are increasingly establishing guidelines and frameworks to ensure the safety and efficacy of diagnostic tools. This regulatory environment fosters innovation and encourages manufacturers to develop advanced diagnostic solutions. Moreover, standardization of diagnostic procedures enhances the reliability of results, which is crucial in critical care scenarios. As regulatory bodies continue to support the development of new technologies, the Critical Care Diagnostics Market is likely to benefit from increased trust and adoption of these innovations, further propelling market growth.
Growing Demand for Personalized Medicine
The shift towards personalized medicine is significantly influencing the Critical Care Diagnostics Market. As healthcare providers aim to tailor treatments to individual patient profiles, the need for precise diagnostic tools becomes paramount. This trend is supported by the increasing availability of genetic and biomarker testing, which enables clinicians to make informed decisions based on a patient's unique characteristics. The market for personalized medicine is expected to reach several billion dollars in the coming years, further driving the demand for advanced diagnostics. Consequently, the Critical Care Diagnostics Market is poised for growth as it aligns with the broader movement towards individualized patient care.
Technological Innovations in Diagnostics
Technological advancements in diagnostic tools are transforming the Critical Care Diagnostics Market. Innovations such as point-of-care testing, telemedicine, and advanced imaging techniques are enhancing the speed and accuracy of diagnostics. For instance, the introduction of portable diagnostic devices allows for immediate testing in critical situations, which is crucial for timely interventions. The market is projected to grow at a compound annual growth rate of around 8% over the next few years, driven by these technological innovations. As healthcare systems increasingly adopt these advanced solutions, the Critical Care Diagnostics Market is likely to witness substantial growth, reflecting the ongoing evolution of diagnostic capabilities.
Aging Population and Increased Healthcare Expenditure
The aging population is a critical factor driving the Critical Care Diagnostics Market. As individuals age, they are more susceptible to various health issues that require intensive care and monitoring. This demographic shift is accompanied by increased healthcare expenditure, as governments and private sectors invest more in healthcare infrastructure and diagnostic technologies. Reports indicate that healthcare spending is projected to rise significantly, reflecting the need for advanced diagnostic solutions in critical care settings. This trend suggests that the Critical Care Diagnostics Market will continue to expand, driven by the dual forces of an aging population and heightened investment in healthcare.