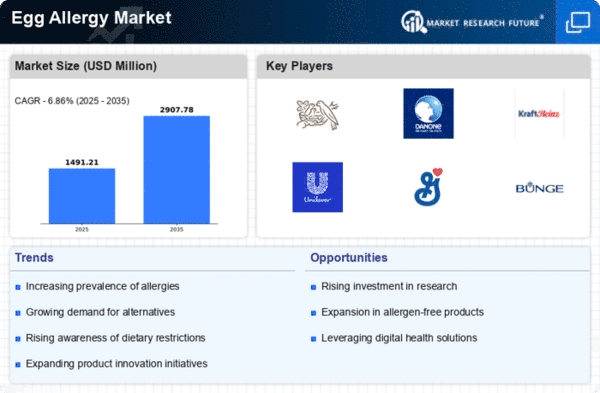
Top Industry Leaders in the Egg Allergy Market

Latest Egg Allergy Companies Update:
Aimmune Therapeutics (US) Announced positive Phase 3 results for its PALFORIA® (peanut immunotherapy) trial in July 2023. This success could pave the way for similar advancements in egg allergy treatment.
DBV Technologies (France) Received FDA approval for its Viaskin Peanut allergy patch in May 2023, marking the first ever approved, non-oral immunotherapy for peanut allergies. This could potentially influence development of similar solutions for egg allergies.
Probiotic Therapeutics (US) Developing LGG-422, a probiotic strain demonstrating potential in preventing and treating egg allergy in early clinical trials. Further research could unlock promising new avenues for managing egg allergies.
Entero Therapeutics (Australia) Launched the Egg Allergen Oral Immunotherapy (EAOI) clinical trial in August 2023, investigating the potential of using egg whites for oral immunotherapy in children with egg allergies. This trial adds to the growing research efforts towards effective egg allergy treatments.
Nestlé Health Science (Switzerland) Partnered with Aimmune Therapeutics in August 2023 to commercialize PALFORIA® in Europe, expanding access to peanut allergy treatment and potentially setting a precedent for similar collaborations in the egg allergy market.
List of Egg Allergy Key companies in the market
- Genentech, Inc. (the US)
- Sanofi SA (France),
- ImmuneTech (US)
- Quest Diagnostics (US)
- HYCOR Biomedical (US),
- Alletess Medical Laboratory (US)
- Kaleo, Inc. (the US)
- Imutest Ltd (UK)
- Creative Diagnostics (US)
- Impax Laboratories (US)
- Mylan NV (US)