Emergence of Point-of-Care Testing
The rise of point-of-care testing (POCT) is transforming the ivd contract-manufacturing market in the GCC. POCT allows for rapid diagnostic results at the site of patient care, which is increasingly favored by healthcare providers. This shift is driven by the need for timely decision-making in patient management. As a result, contract manufacturers are adapting their production capabilities to meet the growing demand for portable and user-friendly diagnostic devices. The market for POCT is expected to expand significantly, with estimates suggesting a growth rate of around 10% annually. This trend presents opportunities for manufacturers to innovate and develop new products tailored to the needs of healthcare professionals.
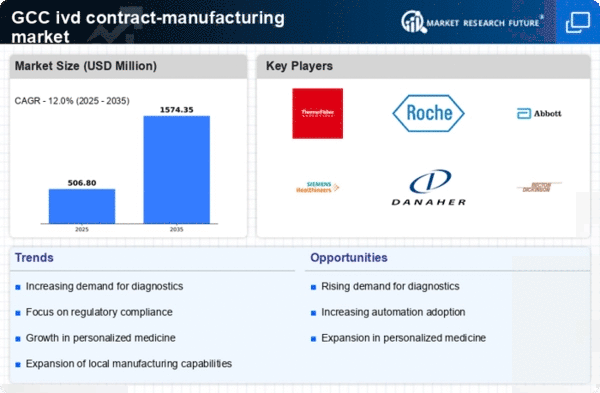
Rising Demand for Diagnostic Testing
The increasing prevalence of chronic diseases and the growing emphasis on preventive healthcare are driving the demand for diagnostic testing in the region. This trend is particularly evident in the GCC, where healthcare systems are evolving to meet the needs of a diverse population. The ivd contract-manufacturing market is experiencing a surge as healthcare providers seek reliable and efficient diagnostic solutions. According to recent data, the market for in vitro diagnostics in the GCC is projected to grow at a CAGR of approximately 8% over the next five years. This growth is likely to create opportunities for contract manufacturers to expand their services and capabilities, catering to the rising demand for innovative diagnostic products.
Growing Focus on Quality and Accuracy
There is a heightened focus on the quality and accuracy of diagnostic tests within the ivd contract-manufacturing market. As healthcare providers and patients demand more reliable results, manufacturers are compelled to adhere to stringent quality standards. This trend is particularly pronounced in the GCC, where regulatory bodies are implementing more rigorous guidelines for diagnostic products. The emphasis on quality assurance is likely to drive innovation and investment in manufacturing processes, ensuring that products meet the highest standards. Consequently, contract manufacturers that prioritize quality may gain a competitive edge in the market, attracting partnerships with healthcare organizations seeking dependable diagnostic solutions.
Investment in Healthcare Infrastructure
The GCC countries are significantly investing in healthcare infrastructure, which is expected to bolster the ivd contract-manufacturing market. Governments are prioritizing the development of advanced healthcare facilities and laboratories, enhancing the capacity for diagnostic testing. For instance, initiatives aimed at improving healthcare access and quality are likely to increase the demand for in vitro diagnostic products. As a result, contract manufacturers may find themselves in a favorable position to partner with healthcare providers and institutions. The total healthcare expenditure in the GCC is anticipated to reach $100 billion by 2025, indicating a robust market environment for ivd contract-manufacturing.
Strategic Collaborations and Partnerships
Strategic collaborations and partnerships are becoming a key driver in the ivd contract-manufacturing market. Companies are increasingly seeking alliances with research institutions, healthcare providers, and technology firms to enhance their product offerings and expand their market reach. In the GCC, such collaborations can facilitate access to advanced technologies and expertise, enabling manufacturers to develop cutting-edge diagnostic solutions. This trend is likely to foster innovation and accelerate the development of new products, as companies leverage each other's strengths. The collaborative approach may also help mitigate risks associated with market entry and product development, positioning contract manufacturers for sustained growth in a competitive landscape.
















Leave a Comment