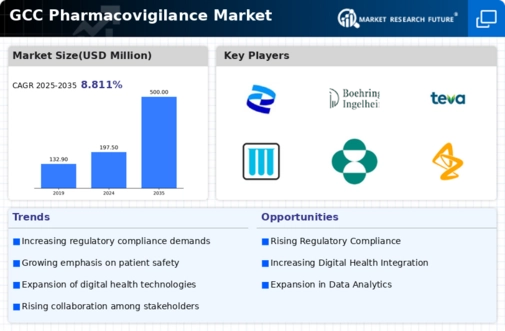
The GCC Pharmacovigilance Market showcases a rapidly evolving landscape characterized by growing regulatory frameworks and increasing awareness surrounding drug safety. As the region experiences heightened healthcare investments, the need for efficient pharmacovigilance activities has surged, leading to the emergence of various players striving to establish a strong foothold.
Competitive dynamics are influenced by factors such as technological advances, the integration of artificial intelligence in monitoring adverse drug reactions, and heightened collaboration between pharmaceutical firms and regulatory bodies.
Entities engaged in this market are continuously adapting to evolving standards, enhancing their data analytics capabilities, and expanding their service offerings to meet diverse customer demands.
Pfizer has established itself as a formidable entity in the GCC Pharmacovigilance Market, leveraging its extensive portfolio of pharmaceutical products and its demonstrated commitment to drug safety. The company's comprehensive pharmacovigilance system ensures thorough monitoring and evaluation of its products, affirming its dedication to patient safety and regulatory compliance.
Pfizer benefits from its strong R&D capabilities and substantial market presence in the GCC, facilitating timely identification and management of adverse events. The company's well-established relationships with local regulatory agencies and a robust network of healthcare professionals further bolster its pharmacovigilance operations.
By maintaining a focus on innovation and regulatory adherence, Pfizer remains a significant player, proactively addressing the unique challenges present in the GCC landscape.
Boehringer Ingelheim also plays a vital role in the GCC Pharmacovigilance Market, with a commitment to ensuring the safety and efficacy of its diverse range of pharmaceuticals, including specialized therapies in areas such as respiratory diseases, diabetes, and oncology.
The company's extensive commitment to pharmacovigilance is supported by its investment in cutting-edge technology and data management systems, which enhance its ability to monitor and analyze safety data effectively. Boehringer Ingelheim's strategic mergers and acquisitions have allowed it to strengthen its capabilities and expand its operational footprint within the GCC region.
The company's strong focus on collaboration with healthcare professionals, combined with its investment in continuous training and education for its pharmacovigilance staff, positions it as a proactive leader in the field. This dedication, coupled with a robust framework for reporting and managing adverse events, reinforces Boehringer Ingelheim's reputable standing in the GCC Pharmacovigilance Market.