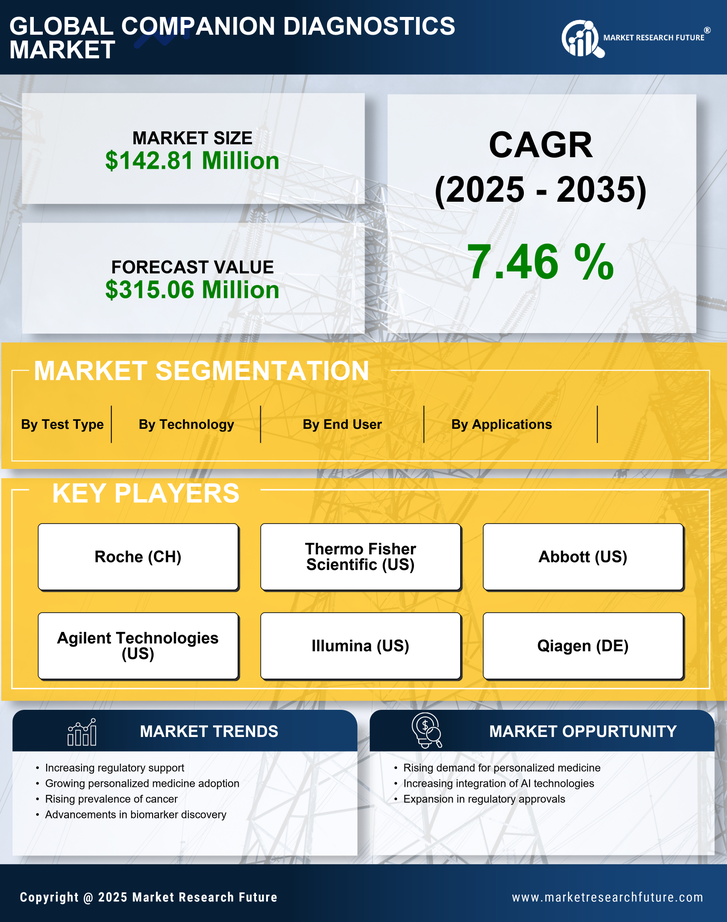
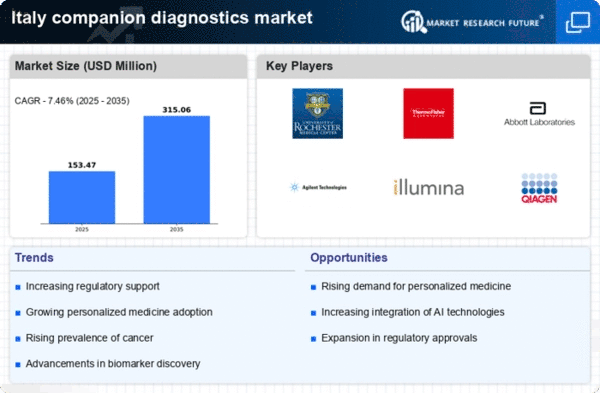
Regulatory Framework Enhancements
The evolving regulatory framework in Italy is playing a crucial role in shaping the companion diagnostics-oncology market. Recent initiatives aimed at streamlining the approval process for diagnostic tests are expected to facilitate quicker market access for innovative products. The Italian Medicines Agency (AIFA) has introduced guidelines that encourage the development of companion diagnostics, thereby fostering collaboration between diagnostic companies and pharmaceutical firms. This regulatory support is likely to enhance the availability of new diagnostic solutions, which are essential for the effective implementation of personalized medicine. As the regulatory landscape continues to evolve, the companion diagnostics-oncology market is poised for growth, driven by increased innovation and market entry of new products.
Growing Investment in Oncology Research
Investment in oncology research is a significant driver for the companion diagnostics-oncology market. In Italy, public and private sectors are allocating substantial funds to cancer research initiatives, which include the development of companion diagnostics. The Italian government has committed over €300 million to cancer research in recent years, fostering innovation in diagnostic tools. This financial support is likely to accelerate the discovery of new biomarkers and the development of personalized therapies. As research progresses, the companion diagnostics-oncology market is expected to benefit from the introduction of novel diagnostic tests that align with emerging treatment modalities, thereby enhancing the overall landscape of cancer care.
Increasing Demand for Targeted Therapies
The rising demand for targeted therapies is a pivotal factor influencing the companion diagnostics-oncology market. In Italy, healthcare professionals are increasingly recognizing the benefits of personalized treatment approaches that align with specific genetic profiles. This shift is reflected in the growing number of targeted therapies approved for various cancer types, which necessitate corresponding companion diagnostics. The market for targeted therapies is anticipated to grow at a CAGR of 8% through 2027, further propelling the need for effective diagnostic tools. As oncologists seek to optimize treatment efficacy, the companion diagnostics-oncology market is likely to expand, driven by the integration of diagnostic tests that support targeted therapy decisions.
Technological Advancements in Diagnostics
the market is experiencing a surge due to rapid technological advancements in diagnostic tools.. Innovations such as next-generation sequencing (NGS) and liquid biopsy techniques are enhancing the precision of cancer diagnostics. In Italy, the integration of these technologies is expected to improve patient outcomes by enabling tailored treatment plans. The market for NGS alone is projected to reach €1.5 billion by 2026, indicating a robust growth trajectory. Furthermore, these advancements facilitate the identification of specific biomarkers, which are crucial for the development of targeted therapies. As healthcare providers increasingly adopt these technologies, the market is likely to expand, driven by the demand for more accurate and efficient diagnostic solutions..
Rising Awareness and Education Initiatives
Rising awareness and education initiatives regarding cancer treatment options are significantly impacting the companion diagnostics-oncology market. In Italy, healthcare organizations are actively promoting the importance of genetic testing and personalized medicine among both healthcare professionals and patients. Educational campaigns are designed to inform stakeholders about the benefits of companion diagnostics in optimizing treatment outcomes. As awareness increases, more patients are likely to seek genetic testing, leading to a higher demand for companion diagnostics. This trend is expected to drive market growth, as healthcare providers recognize the value of integrating diagnostic tests into treatment planning, ultimately enhancing patient care in oncology.