Growing Regulatory Scrutiny
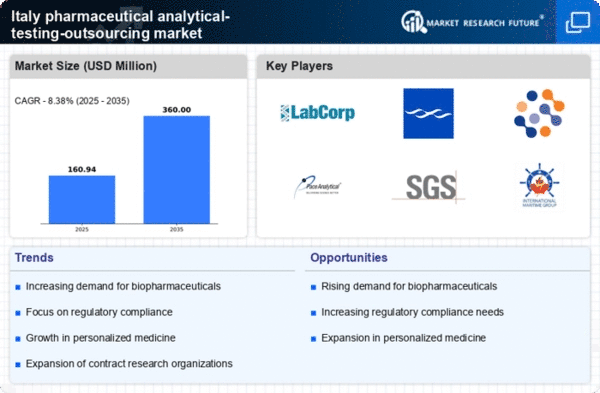
The pharmaceutical industry in Italy is facing heightened regulatory scrutiny, necessitating rigorous testing and compliance measures. This environment has prompted many companies to turn to the pharmaceutical analytical-testing-outsourcing market for specialized services that can ensure adherence to regulatory standards. In 2025, it is anticipated that regulatory compliance costs will account for approximately 20% of total operational expenses for pharmaceutical companies. Consequently, outsourcing analytical testing is seen as a strategic move to mitigate risks and ensure compliance, thereby fostering growth in the outsourcing market.
Emergence of Personalized Medicine
The shift towards personalized medicine in Italy is reshaping the pharmaceutical landscape, creating a demand for tailored analytical testing services. As companies develop targeted therapies, the need for precise and specialized testing becomes paramount. The pharmaceutical analytical-testing-outsourcing market is likely to expand as firms seek external expertise to navigate the complexities of personalized medicine. In 2025, it is projected that the market for personalized medicine will grow by 25%, further driving the need for advanced analytical testing services that can support the development of these innovative therapies.
Rising Demand for Biopharmaceuticals
The increasing prevalence of chronic diseases in Italy has led to a rising demand for biopharmaceuticals, which require extensive analytical testing. The pharmaceutical analytical-testing-outsourcing market is experiencing growth as companies seek to outsource these specialized testing services to ensure compliance with stringent regulations. In 2025, the biopharmaceutical sector is projected to account for approximately 30% of the total pharmaceutical market in Italy, driving the need for reliable analytical testing services. This trend indicates that pharmaceutical companies are likely to invest more in outsourcing to enhance their research and development capabilities, thereby boosting the analytical-testing-outsourcing market.
Focus on Accelerated Drug Development
The pharmaceutical industry in Italy is increasingly focused on accelerating drug development processes to bring new therapies to market more quickly. This urgency has led to a greater reliance on outsourcing analytical testing services, as companies aim to streamline their operations and reduce time-to-market. In 2025, it is estimated that the demand for outsourced analytical testing could grow by 15% annually, reflecting the industry's shift towards more efficient development strategies. The pharmaceutical analytical-testing-outsourcing market is thus positioned to benefit from this trend, as companies seek to leverage external expertise to enhance their development timelines.
Increased Investment in Research and Development
Investment in research and development (R&D) within the pharmaceutical sector in Italy is on the rise, with companies allocating more resources to innovative drug discovery. This trend is expected to drive the pharmaceutical analytical-testing-outsourcing market, as firms require comprehensive testing services to validate their R&D efforts. In 2025, R&D spending in the pharmaceutical industry is projected to reach €3 billion, indicating a robust commitment to innovation. As a result, the demand for outsourced analytical testing services is likely to increase, allowing companies to focus on core competencies while ensuring high-quality testing outcomes.