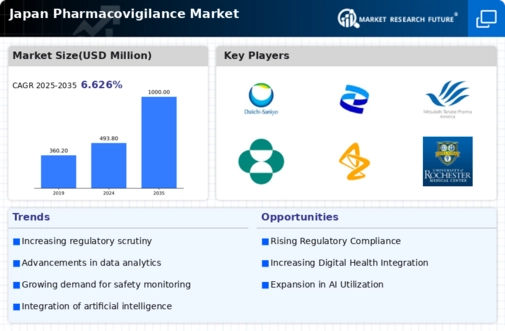
The Japan Pharmacovigilance Market is a critical sector that ensures the safety and efficacy of pharmaceutical products post-marketing. It plays a vital role in monitoring drug safety, collecting data on adverse drug reactions, and conducting risk assessments to protect public health.
The competitive landscape of this market is shaped by various local and global entities striving to enhance their market presence through advanced technologies, regulatory compliance, and comprehensive drug monitoring systems.
As a result, companies in this market must navigate a complex regulatory environment while also focusing on innovative solutions to meet the increasing demands for drug safety and real-time data analysis.
The growing emphasis on patient safety and the rising need for stringent regulatory frameworks further intensify the competition among market players, driving them to invest in robust pharmacovigilance offerings and strategic partnerships.
Daiichi Sankyo stands out in the Japan Pharmacovigilance Market due to its deep-rooted commitment to ensuring drug safety and compliance with regulatory standards. As a leading Japanese pharmaceutical company, it has a well-established pharmacovigilance system that actively monitors and evaluates drug safety profiles, ensuring swift reporting of adverse events and thorough assessment of risks.
The company's strong reputation is reinforced by its extensive research and development capabilities, allowing it to rapidly adapt to changes in regulatory requirements and incorporate advanced technologies into its pharmacovigilance processes.
Daiichi Sankyo is recognized for its robust data management and analysis services, which enhance the efficiency of safety reporting and risk management. This strong foundation enables the company to maintain a prominent position in the pharmacovigilance space within Japan while continuing to build trust with both healthcare professionals and regulatory authorities.
Pfizer also holds a significant position in the Japan Pharmacovigilance Market with its comprehensive suite of services aimed at ensuring drug safety. Known for its leading pharmaceutical products and strong market presence, Pfizer's pharmacovigilance operations in Japan are characterized by advanced technology implementation and a proactive approach to safety monitoring.
The company invests heavily in data analytics and risk management strategies, which help to promptly identify safety concerns associated with its products. Pfizer's strengths lie in its global reach and extensive experience in the pharmaceutical industry, enabling it to effectively leverage international safety data while adhering to local regulatory requirements.
Furthermore, Pfizer's strategic mergers and acquisitions have bolstered its market presence in Japan, allowing the company to diversify its portfolio and enhance its pharmacovigilance capabilities.
By continuously focusing on innovation and compliance, Pfizer remains a formidable competitor in the Japanese pharmacovigilance sector, demonstrating a commitment to safeguarding public health through rigorous monitoring of its pharmaceutical offerings.