Technological Innovations
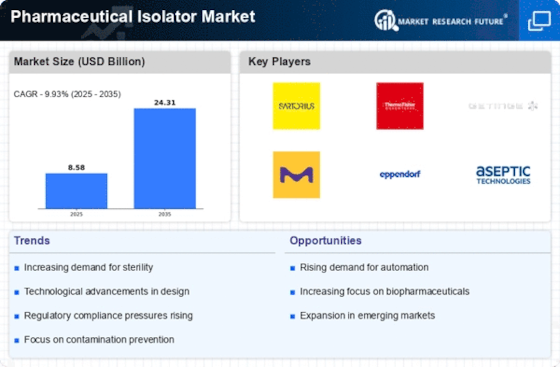
Technological advancements play a pivotal role in shaping the Pharmaceutical Isolator Market. Innovations such as automated isolators and advanced monitoring systems are enhancing the efficiency and reliability of pharmaceutical manufacturing processes. The integration of Internet of Things (IoT) technologies allows for real-time monitoring and data collection, which is crucial for maintaining sterile conditions. Furthermore, the introduction of modular isolators offers flexibility and scalability, catering to the diverse needs of pharmaceutical manufacturers. As companies strive to optimize production while minimizing contamination risks, the adoption of these cutting-edge technologies is expected to increase. Market analysis suggests that the isolator technology segment could witness a compound annual growth rate (CAGR) of approximately 8% over the next five years, underscoring the significance of innovation in the Pharmaceutical Isolator Market.
Stringent Regulatory Frameworks
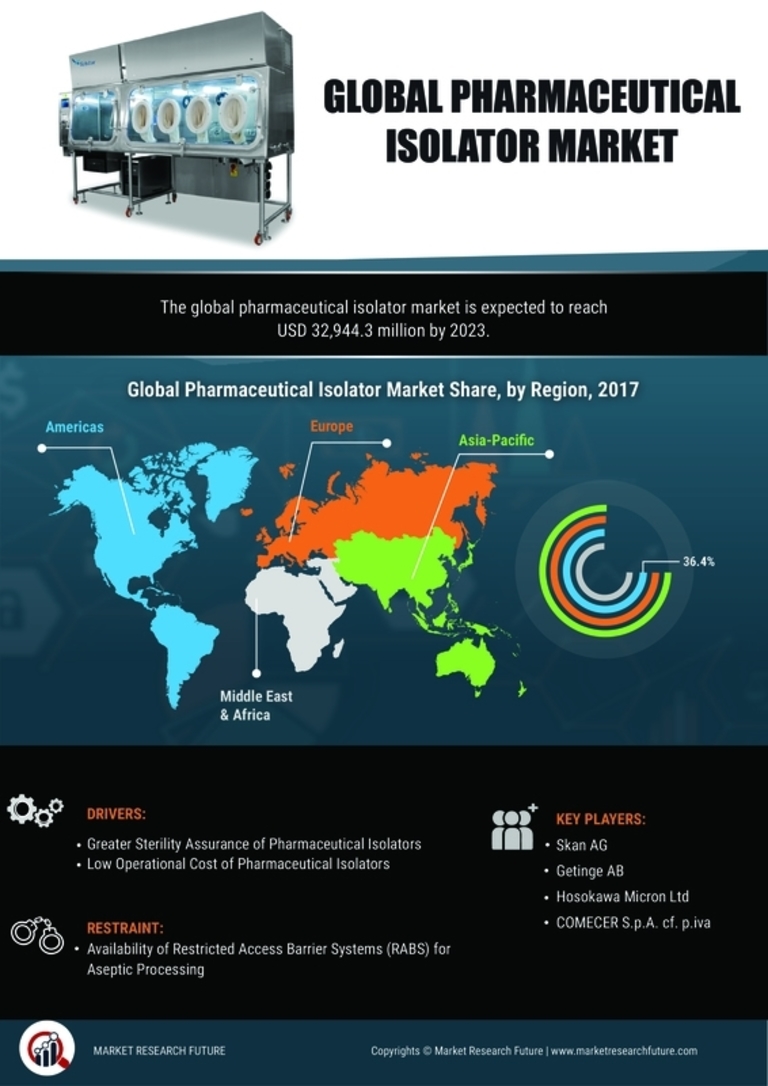
The Pharmaceutical Isolator Market is significantly influenced by stringent regulatory frameworks imposed by health authorities worldwide. Regulatory bodies such as the FDA and EMA enforce rigorous guidelines to ensure the safety and quality of pharmaceutical products. Compliance with these regulations necessitates the use of isolators to maintain aseptic conditions during manufacturing processes. As a result, pharmaceutical companies are compelled to invest in isolator technologies to meet these standards, thereby driving market growth. The increasing frequency of inspections and audits by regulatory agencies further emphasizes the need for robust isolator systems. It is estimated that the compliance costs associated with regulatory requirements could account for up to 20% of total production costs, highlighting the critical role of isolators in the Pharmaceutical Isolator Market.
Growth of Biopharmaceutical Sector
The Pharmaceutical Isolator Market is witnessing substantial growth due to the expansion of the biopharmaceutical sector. Biopharmaceuticals, which include monoclonal antibodies and recombinant proteins, require highly controlled manufacturing environments to prevent contamination. The increasing investment in biopharmaceutical research and development is driving the demand for isolators that can provide the necessary sterile conditions. Market reports indicate that the biopharmaceutical market is expected to reach USD 500 billion by 2025, further propelling the need for advanced isolator solutions. As biopharmaceutical companies prioritize product integrity and safety, the adoption of isolators is likely to become more prevalent, thereby enhancing the overall Pharmaceutical Isolator Market.
Rising Demand for Sterile Products
The Pharmaceutical Isolator Market is experiencing a notable increase in demand for sterile products, driven by the growing need for contamination-free environments in pharmaceutical manufacturing. As the industry evolves, the emphasis on product safety and efficacy has intensified, leading to a surge in the adoption of isolators. According to recent data, the market for sterile pharmaceuticals is projected to reach USD 300 billion by 2026, indicating a robust growth trajectory. This trend is further fueled by the increasing prevalence of chronic diseases, which necessitates the production of sterile injectable drugs. Consequently, pharmaceutical companies are investing in advanced isolator technologies to ensure compliance with stringent quality standards, thereby propelling the Pharmaceutical Isolator Market forward.
Increasing Focus on Quality Assurance
Quality assurance remains a cornerstone of the Pharmaceutical Isolator Market, as companies strive to uphold high standards in product manufacturing. The growing awareness of the importance of quality in pharmaceuticals has led to an increased focus on implementing isolator systems that ensure contamination-free environments. This trend is particularly evident in the production of high-value drugs, where even minor contamination can result in significant financial losses. As a result, pharmaceutical manufacturers are increasingly investing in isolators to enhance their quality assurance processes. Data suggests that companies that prioritize quality assurance can achieve up to 30% higher operational efficiency, indicating the potential benefits of isolator adoption. This focus on quality is expected to drive sustained growth in the Pharmaceutical Isolator Market.