Rising Healthcare Expenditure
The increase in healthcare expenditure across various regions is a significant driver for the Pneumonia Testing Market. As countries allocate more resources to healthcare, there is a corresponding rise in the availability and accessibility of diagnostic services. This trend is particularly evident in developing nations, where investments in healthcare infrastructure are expanding. Enhanced funding allows for the procurement of advanced diagnostic equipment and training of healthcare professionals, which is essential for effective pneumonia testing. Moreover, the emphasis on preventive healthcare is leading to greater awareness and utilization of diagnostic tests, thereby boosting the Pneumonia Testing Market. The correlation between healthcare spending and improved diagnostic capabilities suggests a positive outlook for the market as investments continue to grow.
Increasing Incidence of Pneumonia
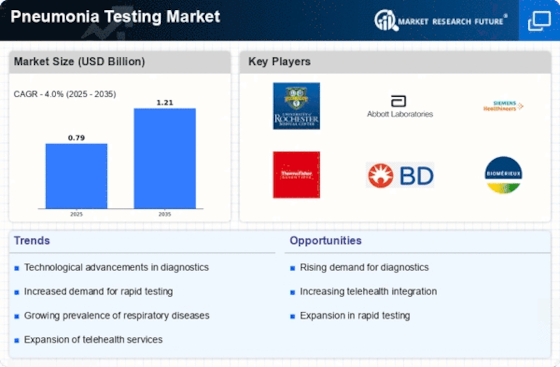
The rising incidence of pneumonia, particularly among vulnerable populations such as the elderly and children, is a primary driver for the Pneumonia Testing Market. According to health statistics, pneumonia remains a leading cause of morbidity and mortality worldwide, with millions of cases reported annually. This trend necessitates enhanced diagnostic capabilities to ensure timely and accurate detection. As healthcare systems strive to manage this burden, the demand for effective pneumonia testing solutions is likely to escalate. Furthermore, the increasing awareness of pneumonia's impact on public health is prompting governments and health organizations to invest in better diagnostic tools, thereby propelling the Pneumonia Testing Market forward. The focus on early detection and treatment is expected to drive innovation and adoption of advanced testing methodologies.
Government Initiatives and Policies
Government initiatives aimed at combating pneumonia are playing a crucial role in shaping the Pneumonia Testing Market. Various health organizations and governments are implementing policies to improve pneumonia diagnosis and treatment, recognizing its public health implications. These initiatives often include funding for research, awareness campaigns, and the establishment of guidelines for pneumonia management. Such policies not only enhance the visibility of pneumonia as a health concern but also encourage the development and adoption of innovative testing solutions. The commitment to reducing pneumonia-related morbidity and mortality rates is likely to drive demand for effective diagnostic tools, thereby positively impacting the Pneumonia Testing Market. Collaborative efforts between governments and private sectors are expected to further accelerate advancements in pneumonia testing.
Growing Awareness of Respiratory Diseases
The increasing awareness of respiratory diseases, including pneumonia, is a significant factor driving the Pneumonia Testing Market. Public health campaigns and educational initiatives are informing populations about the symptoms, risks, and importance of early diagnosis of pneumonia. This heightened awareness is leading to more individuals seeking medical attention and diagnostic testing when experiencing respiratory symptoms. As patients become more informed, the demand for reliable pneumonia testing options is likely to rise. Additionally, healthcare providers are responding to this trend by enhancing their diagnostic capabilities and offering a wider range of testing services. The interplay between public awareness and healthcare responsiveness is expected to foster growth in the Pneumonia Testing Market, as more effective testing solutions become essential in managing respiratory health.
Technological Innovations in Testing Methods
Technological advancements in diagnostic methods are significantly influencing the Pneumonia Testing Market. Innovations such as molecular diagnostics, rapid antigen tests, and imaging techniques are enhancing the accuracy and speed of pneumonia detection. For instance, the introduction of point-of-care testing devices allows for immediate results, which is crucial in emergency settings. The market is witnessing a shift towards more sophisticated testing solutions that can differentiate between bacterial and viral pneumonia, thus guiding appropriate treatment strategies. As healthcare providers increasingly adopt these technologies, the Pneumonia Testing Market is poised for substantial growth. The integration of artificial intelligence and machine learning in diagnostic processes further indicates a trend towards more efficient and reliable testing, potentially transforming patient outcomes.