Regulatory Support and Guidelines
Regulatory frameworks and guidelines established by health authorities play a pivotal role in shaping the hepatitis test-solution-diagnosis market. The US Food and Drug Administration (FDA) has implemented stringent regulations to ensure the safety and efficacy of diagnostic tests. These regulations not only foster innovation but also instill confidence among healthcare providers and patients regarding the reliability of testing solutions. Additionally, the establishment of guidelines for hepatitis screening and diagnosis by organizations such as the CDC further supports the market's growth. These guidelines encourage healthcare providers to adopt standardized testing protocols, thereby increasing the demand for compliant diagnostic solutions. As regulatory support continues to evolve, it is likely to create a conducive environment for the hepatitis test-solution-diagnosis market to thrive.
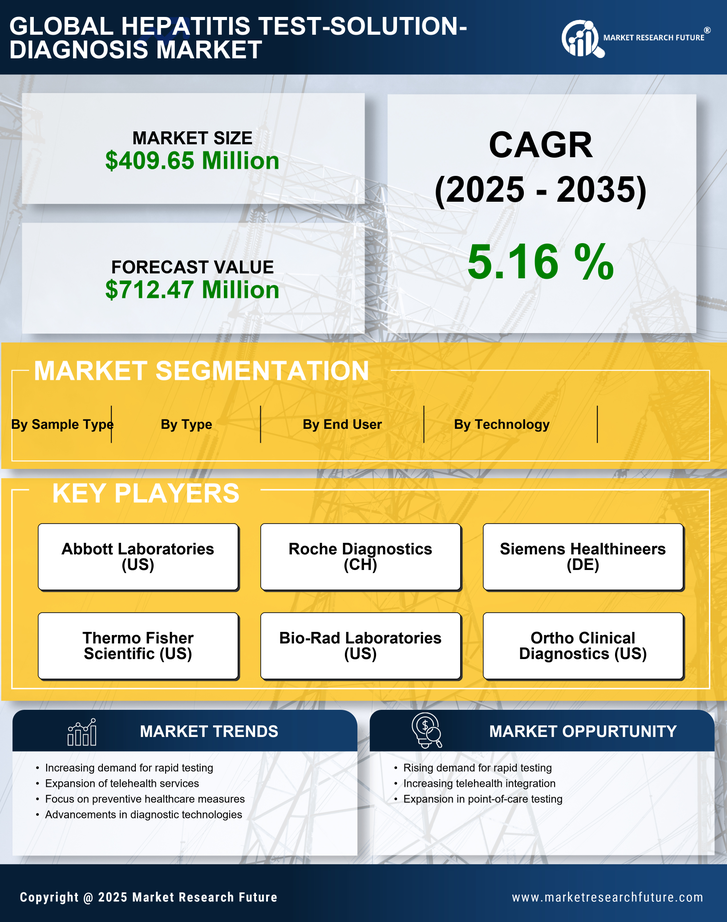
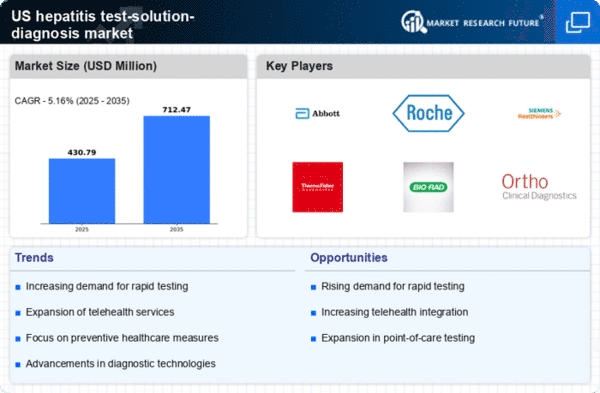
Rising Incidence of Hepatitis Cases
The increasing incidence of hepatitis cases in the US is a critical driver for the hepatitis test-solution-diagnosis market. According to the Centers for Disease Control and Prevention (CDC), hepatitis A, B, and C infections have shown a notable rise in recent years. This trend necessitates enhanced testing solutions to identify and manage these infections effectively. The growing number of reported cases, particularly among high-risk populations, underscores the urgent need for reliable diagnostic tools. As healthcare providers seek to address this public health challenge, the demand for innovative testing solutions is likely to escalate, thereby propelling the market forward. Furthermore, the financial implications of untreated hepatitis infections, which can lead to severe health complications, further emphasize the importance of early detection and diagnosis in the hepatitis test-solution-diagnosis market.
Advancements in Diagnostic Technologies
Technological innovations in diagnostic methods are significantly influencing the hepatitis test-solution-diagnosis market. The introduction of rapid testing kits and molecular diagnostics has revolutionized the way hepatitis infections are detected. These advancements not only enhance the accuracy of diagnoses but also reduce the time required for results, which is crucial for effective patient management. For instance, the development of point-of-care testing devices allows for immediate results, facilitating timely treatment decisions. The market is witnessing a shift towards more sophisticated testing solutions, including next-generation sequencing and automated laboratory systems. As healthcare facilities increasingly adopt these technologies, the hepatitis test-solution-diagnosis market is expected to experience substantial growth, driven by the demand for efficient and reliable diagnostic tools.
Increased Focus on Preventive Healthcare
The growing emphasis on preventive healthcare measures is a significant driver for the hepatitis test-solution-diagnosis market. As healthcare systems in the US prioritize early detection and prevention of diseases, the demand for hepatitis testing solutions is likely to rise. Public health campaigns aimed at educating individuals about the risks associated with hepatitis infections have contributed to a heightened awareness of the importance of regular testing. This shift towards preventive care is reflected in the increasing allocation of resources towards screening programs and diagnostic services. Moreover, the integration of hepatitis testing into routine healthcare check-ups is becoming more common, further driving the market. The proactive approach to managing hepatitis infections aligns with broader public health goals, thereby enhancing the relevance of the hepatitis test-solution-diagnosis market.
Growing Investment in Healthcare Infrastructure
The expansion of healthcare infrastructure in the US is a crucial driver for the hepatitis test-solution-diagnosis market. Increased funding for healthcare facilities, particularly in underserved areas, is enhancing access to diagnostic services. This investment is aimed at improving the overall quality of care and ensuring that individuals have access to necessary testing solutions. As healthcare systems upgrade their facilities and technologies, the demand for advanced hepatitis testing solutions is expected to rise. Furthermore, partnerships between public and private sectors are facilitating the development of innovative diagnostic tools, thereby contributing to market growth. The focus on strengthening healthcare infrastructure aligns with the broader objective of improving public health outcomes, which is likely to benefit the hepatitis test-solution-diagnosis market.