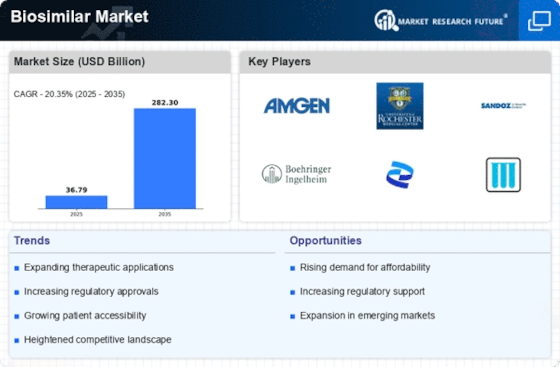
Top Industry Leaders in the Biosimilars Market
 Latest Biosimilars Companies Update
Latest Biosimilars Companies Update
January 2023: the public will be able to purchase a biosimilar version of Humira, a medication for rheumatoid arthritis, from Amgen, the biggest pharmaceutical business in the world. The prescription drug coverage provisions of the customer's health insurance policy will decide the out-of-pocket payments. Even though AbbVie's Humira generated over $20 billion in sales last year, a slew of biosimilars, spearheaded by Amjevita, are set to hit the market this year, posing a serious threat to the popular medicine.
Lannett Company, Inc. updated investors on the research and clinical status of its insulin glargine and insulin aspart biosimilars. For the creation of both items, Lannett formed a strategic collaboration with the HEC Group of Companies (HEC). Subcutaneous administration of equivalent amounts of the reference biologic, US NovoLog®, and the Lannett/HEC biosimilar insulin aspart showed very similar results in a trial conducted by the business.
March 2023: Eli Lilly and Company will launch Rezvoglar, a biosimilar insulin glargine that may be used interchangeably, at a 78% reduction compared to the original (Lantus). Rezvolgar, also known as insulin glargine-aglr, became the fourth biosimilar to be granted interchangeability certification in November 2022. After Semglee (insulin glargine-yfgn), which was approved by the FDA in December 2021, this was the second insulin biosimilar to get the interchangeability label. The insulin lispro reference drug Humalog, the recombinant human insulin Humulin, and an unbranded insulin lispro product are all manufactured by Lilly. Rezvoglar is one of these products.
List of Biosimilars Key companies in the market
-
Pfizer (US)
-
Sandoz (Germany)
-
Biocon (India)
-
Biogen (US)
-
Fresenius Kabi AG (Germany)
-
Boehringer Ingelheim (Germany)
-
Merck KgaA (Germany)
-
Mylan (US)
-
Eli Lilly (US)
-
Teva Pharmaceutical (Israel)