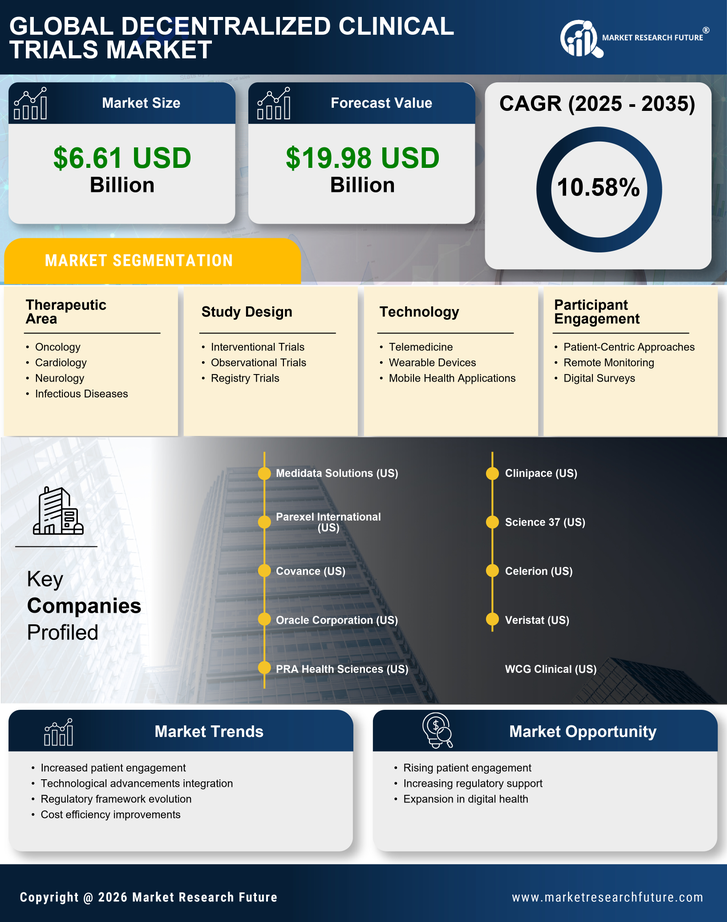
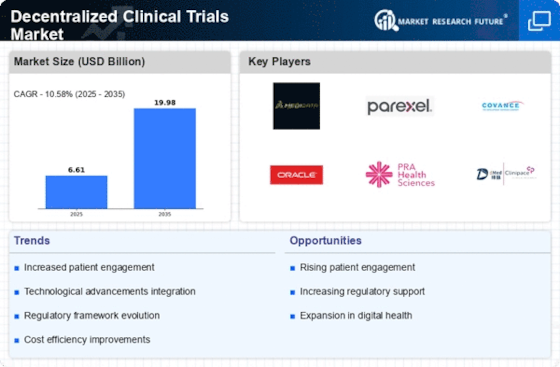
Cost Efficiency
Cost efficiency emerges as a pivotal driver within the Decentralized Clinical Trials Market. Traditional clinical trials often incur substantial expenses related to site management, patient recruitment, and data collection. In contrast, decentralized trials can significantly reduce these costs by minimizing the need for physical sites and streamlining patient monitoring through digital tools. Reports indicate that decentralized trials can lower operational costs by up to 30%, making them an attractive option for sponsors. This financial advantage not only facilitates the initiation of more trials but also allows for the allocation of resources towards innovative research. As the industry continues to evolve, the emphasis on cost efficiency is likely to drive further adoption of decentralized methodologies.
Technological Advancements
Technological advancements are a driving force in the Decentralized Clinical Trials Market. The proliferation of mobile health applications, telemedicine, and remote monitoring devices has revolutionized how clinical trials are conducted. These technologies enable real-time communication between researchers and participants, fostering greater engagement and adherence to trial protocols. Furthermore, the use of artificial intelligence and machine learning in data analysis enhances the efficiency of trial processes, allowing for quicker identification of trends and outcomes. As technology continues to evolve, it is likely to further streamline operations within decentralized trials, making them more appealing to sponsors and participants alike. The ongoing integration of innovative technologies is expected to play a pivotal role in shaping the future landscape of clinical research.
Data-Driven Decision Making
The integration of advanced data analytics is transforming the Decentralized Clinical Trials Market. By utilizing real-time data collection and analysis, researchers can make informed decisions throughout the trial process. This capability enhances the ability to monitor patient safety and efficacy of treatments more effectively. Moreover, the use of electronic health records and wearable devices allows for continuous data flow, which can lead to quicker adjustments in trial protocols if necessary. The potential for improved data integrity and accuracy is significant, as it may reduce the likelihood of errors associated with manual data entry. Consequently, the emphasis on data-driven decision making is likely to propel the growth of decentralized trials, as stakeholders seek to optimize trial outcomes.
Enhanced Patient Accessibility
The Decentralized Clinical Trials Market is witnessing a notable shift towards enhanced patient accessibility. By leveraging digital platforms, these trials allow participants to engage from their homes, thereby reducing geographical barriers. This accessibility is particularly beneficial for patients in remote areas or those with mobility challenges. According to recent data, approximately 70% of patients express a preference for participating in trials that do not require travel. This trend suggests that decentralized trials could potentially increase patient enrollment rates, which is crucial for the timely completion of clinical studies. Furthermore, the ability to reach a more diverse patient population may lead to more comprehensive data collection, ultimately improving the quality of trial outcomes.
Regulatory Support and Guidance
Regulatory support plays a crucial role in shaping the Decentralized Clinical Trials Market. As regulatory bodies recognize the benefits of decentralized methodologies, they are increasingly providing guidance to facilitate their implementation. This support includes the establishment of frameworks that address the unique challenges posed by remote data collection and patient monitoring. For instance, recent initiatives have focused on ensuring data security and patient privacy in decentralized settings. Such regulatory advancements not only enhance the credibility of decentralized trials but also encourage sponsors to adopt these innovative approaches. The ongoing dialogue between regulators and industry stakeholders suggests a growing acceptance of decentralized trials, which may lead to a more favorable environment for their expansion.