Focus on Cost Reduction
Cost reduction remains a pivotal driver in the Lab Automation for In-vitro Diagnostic Market. As healthcare providers strive to manage budgets while maintaining quality, automation presents a viable solution. By minimizing manual labor and optimizing workflows, laboratories can significantly lower operational costs. Market analysis reveals that automated systems can reduce testing costs by up to 30%, making them an attractive investment for many facilities. This focus on cost efficiency is further amplified by the increasing competition among diagnostic laboratories, compelling them to adopt automation technologies to enhance their service offerings and remain competitive.
Regulatory Support for Automation
Regulatory bodies are increasingly recognizing the benefits of automation in the Lab Automation for In-vitro Diagnostic Market. Supportive regulations and guidelines are being established to encourage the adoption of automated systems, which are seen as essential for improving diagnostic accuracy and efficiency. This regulatory backing not only fosters innovation but also instills confidence among stakeholders regarding the safety and efficacy of automated solutions. As a result, laboratories are more inclined to invest in automation technologies, knowing that they are aligned with regulatory expectations and standards, thus facilitating smoother market entry for new products.
Integration of Advanced Technologies
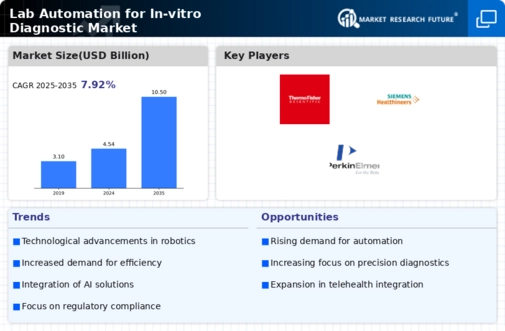
The Lab Automation for In-vitro Diagnostic Market is experiencing a notable shift towards the integration of advanced technologies such as robotics and artificial intelligence. These technologies enhance the efficiency and accuracy of diagnostic processes, thereby reducing human error. The market is projected to grow significantly, with estimates suggesting a compound annual growth rate of over 10% in the coming years. This growth is driven by the increasing demand for high-throughput testing and the need for rapid results in clinical settings. As laboratories seek to streamline operations, the adoption of automated systems becomes essential, allowing for better resource management and improved patient outcomes.
Growing Emphasis on Patient-Centric Care
The Lab Automation for In-vitro Diagnostic Market is increasingly aligning with the trend towards patient-centric care. As healthcare systems evolve, there is a growing emphasis on delivering personalized and timely diagnostic services. Automation plays a crucial role in this transformation by enabling laboratories to provide faster turnaround times and more accurate results. This shift is supported by market data indicating that patient satisfaction is closely linked to the efficiency of diagnostic processes. Consequently, laboratories are investing in automation technologies to enhance their service delivery, ultimately improving patient outcomes and fostering loyalty in an increasingly competitive landscape.
Rising Demand for High-Throughput Testing
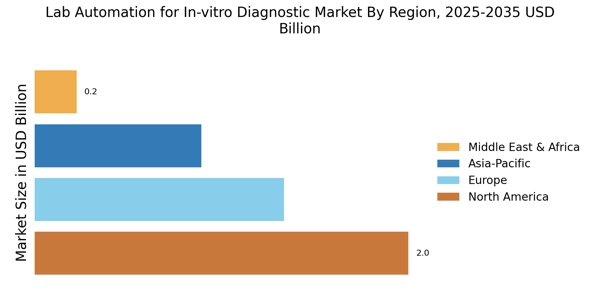
The Lab Automation for In-vitro Diagnostic Market is witnessing a surge in demand for high-throughput testing capabilities. This trend is largely influenced by the increasing prevalence of chronic diseases and the need for timely diagnosis. Laboratories are under pressure to process a higher volume of samples efficiently, which necessitates the implementation of automated solutions. Market data indicates that the high-throughput segment is expected to account for a substantial share of the overall market, driven by advancements in technology that facilitate faster and more accurate testing. Consequently, automation solutions that cater to this demand are becoming increasingly vital for laboratories.