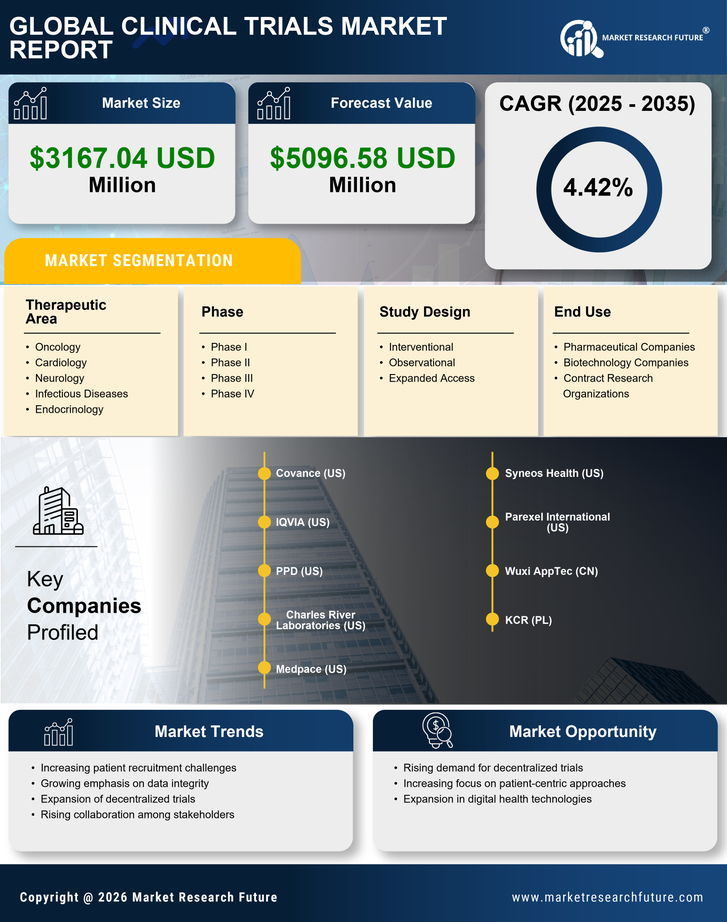
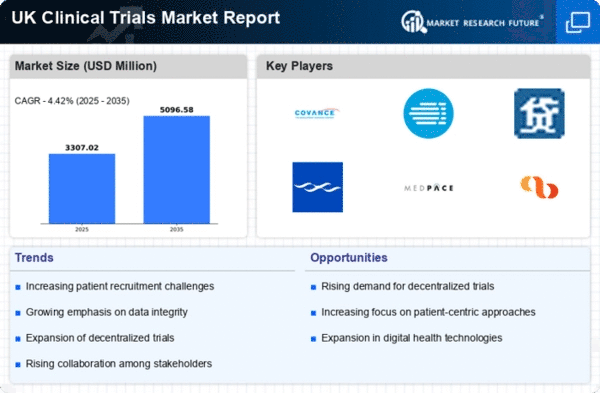
The clinical trials market in the UK is characterized by a dynamic competitive landscape, driven by innovation, regulatory changes, and the increasing demand for efficient drug development processes. Key players such as Covance (US), IQVIA (US), and Charles River Laboratories (US) are at the forefront, employing diverse strategies to enhance their market positioning. Covance (US) focuses on integrating advanced technologies into its trial processes, which appears to streamline operations and improve data accuracy. Meanwhile, IQVIA (US) emphasizes partnerships with biotech firms, leveraging its extensive data analytics capabilities to optimize trial designs and patient recruitment. Charles River Laboratories (US) is actively pursuing regional expansion, particularly in the UK, to capitalize on the growing demand for preclinical and clinical services, thereby enhancing its competitive edge. The market structure is moderately fragmented, with a mix of large multinational corporations and smaller specialized firms. Key players are increasingly localizing their operations to better serve regional markets, which may enhance supply chain efficiency and responsiveness. This localization strategy, combined with supply chain optimization, is likely to strengthen their competitive positions. The collective influence of these companies shapes a landscape where agility and adaptability are paramount, as they navigate regulatory complexities and evolving client needs. In October 2025, Covance (US) announced a strategic partnership with a leading UK-based biotech firm to co-develop a new platform for real-time patient monitoring during clinical trials. This initiative is expected to enhance patient engagement and data collection, potentially leading to faster trial outcomes. The strategic importance of this partnership lies in its ability to leverage Covance's technological expertise while addressing the specific needs of the UK market, thereby positioning the company as a leader in innovative trial methodologies. In September 2025, IQVIA (US) launched a new AI-driven analytics tool designed to improve patient recruitment and retention in clinical trials. This tool utilizes machine learning algorithms to identify suitable candidates more efficiently, which could significantly reduce trial timelines. The introduction of this technology underscores IQVIA's commitment to digital transformation and its potential to reshape the patient recruitment landscape, making trials more accessible and efficient. In August 2025, Charles River Laboratories (US) expanded its facilities in the UK, investing approximately £20 million to enhance its preclinical and clinical research capabilities. This expansion is strategically significant as it not only increases the company's operational capacity but also reinforces its commitment to the UK market, where demand for clinical trial services is on the rise. Such investments are likely to bolster Charles River's competitive position by enabling it to offer a broader range of services to clients. As of November 2025, the clinical trials market is witnessing trends such as digitalization, sustainability, and the integration of AI technologies. These trends are reshaping competitive dynamics, with companies increasingly forming strategic alliances to enhance their service offerings and operational efficiencies. The shift from price-based competition to a focus on innovation, technology, and supply chain reliability is becoming evident. Moving forward, competitive differentiation will likely hinge on the ability to leverage advanced technologies and foster collaborative partnerships, ensuring that companies remain agile and responsive to the evolving demands of the clinical trials landscape.