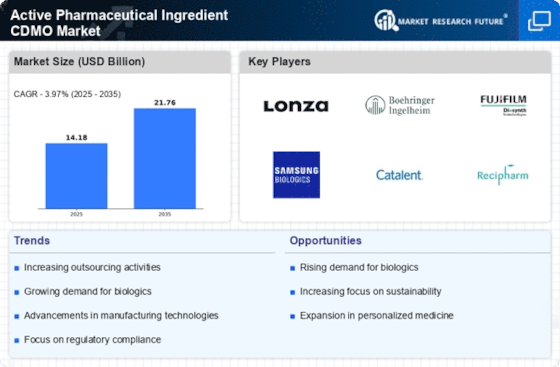
Rising Demand for Biologics
The increasing prevalence of chronic diseases and the aging population are driving the demand for biologics, which are complex molecules derived from living organisms. This trend is particularly evident in the Active Pharmaceutical Ingredient CDMO Market, where the production of biologics requires specialized manufacturing capabilities. According to recent data, the biologics segment is projected to grow at a compound annual growth rate of over 8% in the coming years. As pharmaceutical companies seek to outsource the production of these complex molecules, CDMOs that specialize in biologics are likely to see a surge in demand. This shift not only enhances the capabilities of pharmaceutical companies but also allows for more efficient resource allocation, thereby fostering innovation in drug development.
Increased Focus on Cost Efficiency
Pharmaceutical companies are increasingly seeking ways to reduce operational costs while maintaining high-quality standards. This focus on cost efficiency is a significant driver in the Active Pharmaceutical Ingredient CDMO Market. By outsourcing production to CDMOs, companies can leverage economies of scale and specialized expertise, which can lead to substantial cost savings. Recent analyses indicate that outsourcing can reduce production costs by up to 30%, allowing pharmaceutical firms to allocate resources more effectively. As competition intensifies, the ability to produce high-quality APIs at lower costs becomes a critical factor for success. Consequently, CDMOs that can offer competitive pricing without compromising quality are likely to thrive in this evolving landscape.
Growing Interest in Personalized Medicine
The shift towards personalized medicine is becoming a pivotal trend in the Active Pharmaceutical Ingredient CDMO Market. As healthcare moves towards more tailored treatment options, the demand for APIs that cater to specific patient needs is increasing. This trend is particularly relevant in the development of targeted therapies and precision medicine, which require specialized manufacturing processes. CDMOs that can adapt to these evolving requirements are likely to see increased demand for their services. Market data suggests that the personalized medicine segment is expected to grow significantly, with projections indicating a market size of over 2 trillion dollars by 2030. This growth presents a substantial opportunity for CDMOs to position themselves as leaders in the production of customized APIs, thereby enhancing their market presence.
Technological Innovations in Manufacturing
Advancements in manufacturing technologies are reshaping the landscape of the Active Pharmaceutical Ingredient CDMO Market. Innovations such as continuous manufacturing, process analytical technology, and automation are enhancing production efficiency and reducing time-to-market for new drugs. These technologies enable CDMOs to optimize their processes, resulting in higher yields and lower production costs. For instance, continuous manufacturing can reduce production time by up to 50%, allowing for a more agile response to market demands. As pharmaceutical companies increasingly seek partners that can leverage these technological advancements, CDMOs that invest in state-of-the-art manufacturing capabilities are likely to gain a competitive edge. This trend not only benefits CDMOs but also accelerates the overall drug development process.
Regulatory Compliance and Quality Assurance
The stringent regulatory environment surrounding pharmaceutical manufacturing necessitates a robust approach to quality assurance and compliance. In the Active Pharmaceutical Ingredient CDMO Market, adherence to regulatory standards is paramount for ensuring product safety and efficacy. CDMOs that invest in quality management systems and maintain compliance with international regulations are better positioned to attract clients. The market is witnessing an increasing emphasis on Good Manufacturing Practices (GMP), which are essential for maintaining product integrity. As regulatory bodies continue to tighten their oversight, the demand for CDMOs that can demonstrate a commitment to quality and compliance is expected to rise. This trend not only enhances the reputation of CDMOs but also fosters trust among pharmaceutical companies.