Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Intravenous
Subcutaneous
Intramuscular
North America
Europe
South America
Asia Pacific
Middle East and Africa
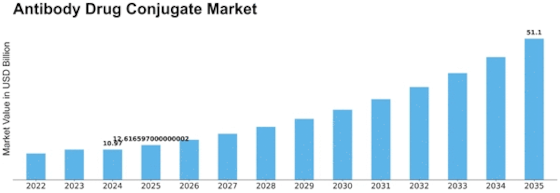
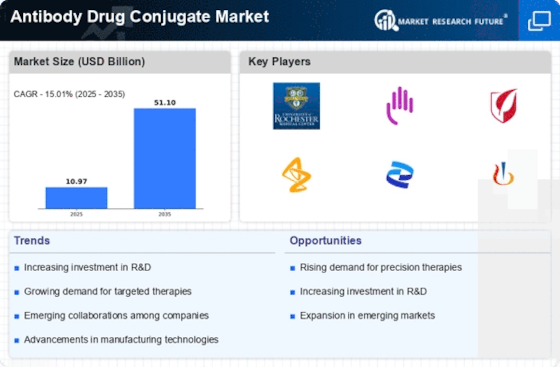
North America Outlook (USD Billion, 2019-2035)
Antibody Drug Conjugate Market by Type
Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Antibody Drug Conjugate Market by Therapeutic Area Type
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Antibody Drug Conjugate Market by Mechanism of Action Type
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Antibody Drug Conjugate Market by Route of Administration Type
Intravenous
Subcutaneous
Intramuscular
Antibody Drug Conjugate Market by Regional Type
US
Canada
US Outlook (USD Billion, 2019-2035)
Antibody Drug Conjugate Market by Type
Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Antibody Drug Conjugate Market by Therapeutic Area Type
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Antibody Drug Conjugate Market by Mechanism of Action Type
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Antibody Drug Conjugate Market by Route of Administration Type
Intravenous
Subcutaneous
Intramuscular
CANADA Outlook (USD Billion, 2019-2035)
Antibody Drug Conjugate Market by Type
Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Antibody Drug Conjugate Market by Therapeutic Area Type
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Antibody Drug Conjugate Market by Mechanism of Action Type
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Antibody Drug Conjugate Market by Route of Administration Type
Intravenous
Subcutaneous
Intramuscular
Europe Outlook (USD Billion, 2019-2035)
Antibody Drug Conjugate Market by Type
Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Antibody Drug Conjugate Market by Therapeutic Area Type
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Antibody Drug Conjugate Market by Mechanism of Action Type
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Antibody Drug Conjugate Market by Route of Administration Type
Intravenous
Subcutaneous
Intramuscular
Antibody Drug Conjugate Market by Regional Type
Germany
UK
France
Russia
Italy
Spain
Rest of Europe
GERMANY Outlook (USD Billion, 2019-2035)
Antibody Drug Conjugate Market by Type
Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Antibody Drug Conjugate Market by Therapeutic Area Type
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Antibody Drug Conjugate Market by Mechanism of Action Type
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Antibody Drug Conjugate Market by Route of Administration Type
Intravenous
Subcutaneous
Intramuscular
UK Outlook (USD Billion, 2019-2035)
Antibody Drug Conjugate Market by Type
Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Antibody Drug Conjugate Market by Therapeutic Area Type
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Antibody Drug Conjugate Market by Mechanism of Action Type
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Antibody Drug Conjugate Market by Route of Administration Type
Intravenous
Subcutaneous
Intramuscular
FRANCE Outlook (USD Billion, 2019-2035)
Antibody Drug Conjugate Market by Type
Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Antibody Drug Conjugate Market by Therapeutic Area Type
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Antibody Drug Conjugate Market by Mechanism of Action Type
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Antibody Drug Conjugate Market by Route of Administration Type
Intravenous
Subcutaneous
Intramuscular
RUSSIA Outlook (USD Billion, 2019-2035)
Antibody Drug Conjugate Market by Type
Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Antibody Drug Conjugate Market by Therapeutic Area Type
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Antibody Drug Conjugate Market by Mechanism of Action Type
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Antibody Drug Conjugate Market by Route of Administration Type
Intravenous
Subcutaneous
Intramuscular
ITALY Outlook (USD Billion, 2019-2035)
Antibody Drug Conjugate Market by Type
Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Antibody Drug Conjugate Market by Therapeutic Area Type
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Antibody Drug Conjugate Market by Mechanism of Action Type
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Antibody Drug Conjugate Market by Route of Administration Type
Intravenous
Subcutaneous
Intramuscular
SPAIN Outlook (USD Billion, 2019-2035)
Antibody Drug Conjugate Market by Type
Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Antibody Drug Conjugate Market by Therapeutic Area Type
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Antibody Drug Conjugate Market by Mechanism of Action Type
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Antibody Drug Conjugate Market by Route of Administration Type
Intravenous
Subcutaneous
Intramuscular
REST OF EUROPE Outlook (USD Billion, 2019-2035)
Antibody Drug Conjugate Market by Type
Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Antibody Drug Conjugate Market by Therapeutic Area Type
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Antibody Drug Conjugate Market by Mechanism of Action Type
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Antibody Drug Conjugate Market by Route of Administration Type
Intravenous
Subcutaneous
Intramuscular
APAC Outlook (USD Billion, 2019-2035)
Antibody Drug Conjugate Market by Type
Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Antibody Drug Conjugate Market by Therapeutic Area Type
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Antibody Drug Conjugate Market by Mechanism of Action Type
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Antibody Drug Conjugate Market by Route of Administration Type
Intravenous
Subcutaneous
Intramuscular
Antibody Drug Conjugate Market by Regional Type
China
India
Japan
South Korea
Malaysia
Thailand
Indonesia
Rest of APAC
CHINA Outlook (USD Billion, 2019-2035)
Antibody Drug Conjugate Market by Type
Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Antibody Drug Conjugate Market by Therapeutic Area Type
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Antibody Drug Conjugate Market by Mechanism of Action Type
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Antibody Drug Conjugate Market by Route of Administration Type
Intravenous
Subcutaneous
Intramuscular
INDIA Outlook (USD Billion, 2019-2035)
Antibody Drug Conjugate Market by Type
Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Antibody Drug Conjugate Market by Therapeutic Area Type
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Antibody Drug Conjugate Market by Mechanism of Action Type
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Antibody Drug Conjugate Market by Route of Administration Type
Intravenous
Subcutaneous
Intramuscular
JAPAN Outlook (USD Billion, 2019-2035)
Antibody Drug Conjugate Market by Type
Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Antibody Drug Conjugate Market by Therapeutic Area Type
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Antibody Drug Conjugate Market by Mechanism of Action Type
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Antibody Drug Conjugate Market by Route of Administration Type
Intravenous
Subcutaneous
Intramuscular
SOUTH KOREA Outlook (USD Billion, 2019-2035)
Antibody Drug Conjugate Market by Type
Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Antibody Drug Conjugate Market by Therapeutic Area Type
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Antibody Drug Conjugate Market by Mechanism of Action Type
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Antibody Drug Conjugate Market by Route of Administration Type
Intravenous
Subcutaneous
Intramuscular
MALAYSIA Outlook (USD Billion, 2019-2035)
Antibody Drug Conjugate Market by Type
Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Antibody Drug Conjugate Market by Therapeutic Area Type
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Antibody Drug Conjugate Market by Mechanism of Action Type
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Antibody Drug Conjugate Market by Route of Administration Type
Intravenous
Subcutaneous
Intramuscular
THAILAND Outlook (USD Billion, 2019-2035)
Antibody Drug Conjugate Market by Type
Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Antibody Drug Conjugate Market by Therapeutic Area Type
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Antibody Drug Conjugate Market by Mechanism of Action Type
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Antibody Drug Conjugate Market by Route of Administration Type
Intravenous
Subcutaneous
Intramuscular
INDONESIA Outlook (USD Billion, 2019-2035)
Antibody Drug Conjugate Market by Type
Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Antibody Drug Conjugate Market by Therapeutic Area Type
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Antibody Drug Conjugate Market by Mechanism of Action Type
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Antibody Drug Conjugate Market by Route of Administration Type
Intravenous
Subcutaneous
Intramuscular
REST OF APAC Outlook (USD Billion, 2019-2035)
Antibody Drug Conjugate Market by Type
Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Antibody Drug Conjugate Market by Therapeutic Area Type
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Antibody Drug Conjugate Market by Mechanism of Action Type
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Antibody Drug Conjugate Market by Route of Administration Type
Intravenous
Subcutaneous
Intramuscular
South America Outlook (USD Billion, 2019-2035)
Antibody Drug Conjugate Market by Type
Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Antibody Drug Conjugate Market by Therapeutic Area Type
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Antibody Drug Conjugate Market by Mechanism of Action Type
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Antibody Drug Conjugate Market by Route of Administration Type
Intravenous
Subcutaneous
Intramuscular
Antibody Drug Conjugate Market by Regional Type
Brazil
Mexico
Argentina
Rest of South America
BRAZIL Outlook (USD Billion, 2019-2035)
Antibody Drug Conjugate Market by Type
Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Antibody Drug Conjugate Market by Therapeutic Area Type
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Antibody Drug Conjugate Market by Mechanism of Action Type
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Antibody Drug Conjugate Market by Route of Administration Type
Intravenous
Subcutaneous
Intramuscular
MEXICO Outlook (USD Billion, 2019-2035)
Antibody Drug Conjugate Market by Type
Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Antibody Drug Conjugate Market by Therapeutic Area Type
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Antibody Drug Conjugate Market by Mechanism of Action Type
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Antibody Drug Conjugate Market by Route of Administration Type
Intravenous
Subcutaneous
Intramuscular
ARGENTINA Outlook (USD Billion, 2019-2035)
Antibody Drug Conjugate Market by Type
Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Antibody Drug Conjugate Market by Therapeutic Area Type
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Antibody Drug Conjugate Market by Mechanism of Action Type
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Antibody Drug Conjugate Market by Route of Administration Type
Intravenous
Subcutaneous
Intramuscular
REST OF SOUTH AMERICA Outlook (USD Billion, 2019-2035)
Antibody Drug Conjugate Market by Type
Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Antibody Drug Conjugate Market by Therapeutic Area Type
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Antibody Drug Conjugate Market by Mechanism of Action Type
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Antibody Drug Conjugate Market by Route of Administration Type
Intravenous
Subcutaneous
Intramuscular
MEA Outlook (USD Billion, 2019-2035)
Antibody Drug Conjugate Market by Type
Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Antibody Drug Conjugate Market by Therapeutic Area Type
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Antibody Drug Conjugate Market by Mechanism of Action Type
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Antibody Drug Conjugate Market by Route of Administration Type
Intravenous
Subcutaneous
Intramuscular
Antibody Drug Conjugate Market by Regional Type
GCC Countries
South Africa
Rest of MEA
GCC COUNTRIES Outlook (USD Billion, 2019-2035)
Antibody Drug Conjugate Market by Type
Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Antibody Drug Conjugate Market by Therapeutic Area Type
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Antibody Drug Conjugate Market by Mechanism of Action Type
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Antibody Drug Conjugate Market by Route of Administration Type
Intravenous
Subcutaneous
Intramuscular
SOUTH AFRICA Outlook (USD Billion, 2019-2035)
Antibody Drug Conjugate Market by Type
Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Antibody Drug Conjugate Market by Therapeutic Area Type
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Antibody Drug Conjugate Market by Mechanism of Action Type
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Antibody Drug Conjugate Market by Route of Administration Type
Intravenous
Subcutaneous
Intramuscular
REST OF MEA Outlook (USD Billion, 2019-2035)
Antibody Drug Conjugate Market by Type
Cytotoxic Drug Conjugates
Immunomodulatory Drug Conjugates
Radiolabeled Drug Conjugates
Dual Action Drug Conjugates
Antibody Drug Conjugate Market by Therapeutic Area Type
Oncology
Autoimmune Diseases
Infectious Diseases
Neurological Disorders
Antibody Drug Conjugate Market by Mechanism of Action Type
Targeted Delivery
Cell Cycle Disruption
Immune Modulation
Antibody-Dependent Cellular Cytotoxicity
Antibody Drug Conjugate Market by Route of Administration Type
Intravenous
Subcutaneous
Intramuscular


















Leave a Comment