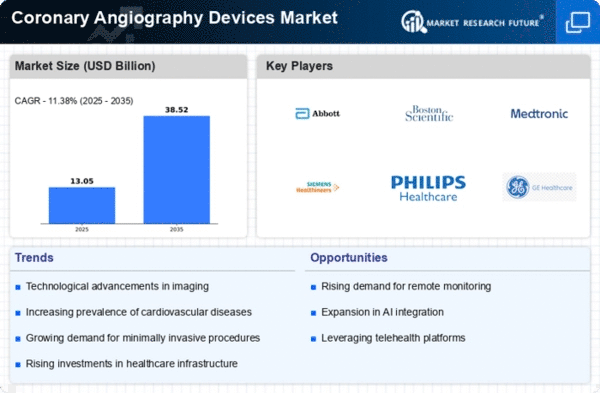
Top Industry Leaders in the Coronary Angiography Devices Market

Latest Coronary angiography devices Companies Updates
Abbott Laboratories Secured FDA approval for their SENTINEL Cerebral Protection System, designed to capture debris during TAVR and potentially reduce stroke risk.Partnered with leading academic centers to conduct research on the effectiveness of their EPDs in various vascular interventions.
Boston Scientific Corporation Launched their Emboshield™ distal protection filter for capturing debris during peripheral vascular procedures.Reported positive results from a clinical trial evaluating their ACORN distal occlusion device for preventing stroke during TAVR.
Medtronic plc Collaborated with a startup company to develop a next-generation EPD with enhanced conformability and adaptability to complex vascular anatomy.Introduced their Embolic Defense™ Embolic Protection System for capturing debris during lower extremity angioplasty and stenting.
Terumo Corporation Received CE Mark approval for their TriGuard™ HD Cerebral Protection System, offering a compact and easy-to-use option for protecting patients during TAVR.Focused on developing bioresorbable EPDs that dissolve after the procedure, potentially reducing long-term complications.
Cook Medical Acquired a smaller EPD technology company, expanding their portfolio and expertise in the coronary EPD market.Introduced their Zenith™ Flex distal protection filter with a unique design for improved deliverability and capture efficiency.
List of Coronary Angiography Devices Key companies in the market
- Terumo Medical Corporation
- Boston Scientific Corporation
- Canon Medical Systems
- Cordis (A Cardinal Health Company)
- Braun Melsungen AG
- Angiodynamics
- Abbott Vascular (Abbott Laboratories)
- Toshiba Medical Systems Corporation
- Royal Philips Electronics
- GE Healthcare
- Medtronic, Inc.
- Shimadzu Corporation
- Siemens Healthcare