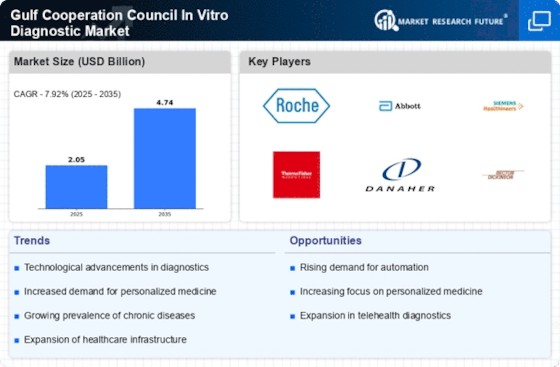
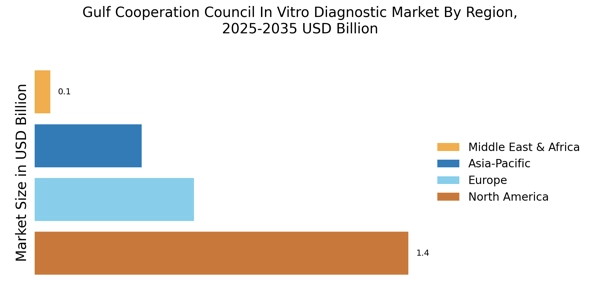
Rising Prevalence of Chronic Diseases
The Gulf Cooperation Council In Vitro Diagnostic Market is experiencing a notable surge due to the increasing prevalence of chronic diseases such as diabetes, cardiovascular disorders, and cancer. As healthcare systems in the region strive to manage these conditions effectively, the demand for in vitro diagnostic (IVD) tests is likely to rise. According to recent estimates, chronic diseases account for a significant portion of healthcare expenditures in the GCC, prompting governments to invest in advanced diagnostic technologies. This trend not only enhances patient outcomes but also drives the growth of the IVD market, as healthcare providers seek efficient and accurate diagnostic solutions to address the rising burden of chronic illnesses.
Investment in Healthcare Infrastructure
The Gulf Cooperation Council In Vitro Diagnostic Market is benefiting from substantial investments in healthcare infrastructure across the region. Governments are prioritizing the development of advanced healthcare facilities and laboratories, which are essential for the effective implementation of in vitro diagnostic technologies. This investment trend is likely to enhance the capacity for diagnostic testing, thereby improving patient care and outcomes. Recent reports indicate that healthcare spending in the GCC is projected to increase significantly, with a focus on modernizing diagnostic capabilities. As a result, the IVD market is poised for growth, driven by the establishment of state-of-the-art facilities and the integration of cutting-edge diagnostic tools.
Growing Demand for Point-of-Care Testing
The Gulf Cooperation Council In Vitro Diagnostic Market is witnessing a shift towards point-of-care testing (POCT), which offers rapid results and convenience for patients. This trend is particularly relevant in the context of the region's healthcare landscape, where timely diagnosis is crucial for effective treatment. The increasing adoption of POCT devices is driven by the need for immediate decision-making in clinical settings, especially in emergency care and remote areas. Market data suggests that the POCT segment is expected to grow at a robust rate, reflecting the region's commitment to enhancing healthcare accessibility and efficiency through innovative diagnostic solutions.
Increasing Awareness of Early Disease Detection
The Gulf Cooperation Council In Vitro Diagnostic Market is experiencing growth due to heightened awareness regarding the importance of early disease detection. Public health campaigns and educational initiatives are fostering a culture of preventive healthcare, encouraging individuals to undergo regular diagnostic testing. This shift in mindset is likely to drive demand for a wide range of in vitro diagnostic tests, as early detection is crucial for effective treatment and management of diseases. Market analysis indicates that as awareness increases, the adoption of IVD solutions is expected to rise, further propelling the growth of the market in the GCC region.
Technological Innovations in Diagnostic Solutions
The Gulf Cooperation Council In Vitro Diagnostic Market is significantly influenced by ongoing technological innovations in diagnostic solutions. Advancements in molecular diagnostics, automation, and digital health technologies are transforming the landscape of in vitro diagnostics. These innovations not only enhance the accuracy and speed of diagnostic tests but also facilitate the integration of IVD solutions into routine clinical practice. Market trends suggest that the introduction of novel diagnostic platforms and technologies is likely to drive competition among key players, ultimately benefiting healthcare providers and patients alike. As the region embraces these advancements, the IVD market is expected to expand, reflecting the growing demand for sophisticated diagnostic capabilities.