Rising Prevalence of Chronic Diseases
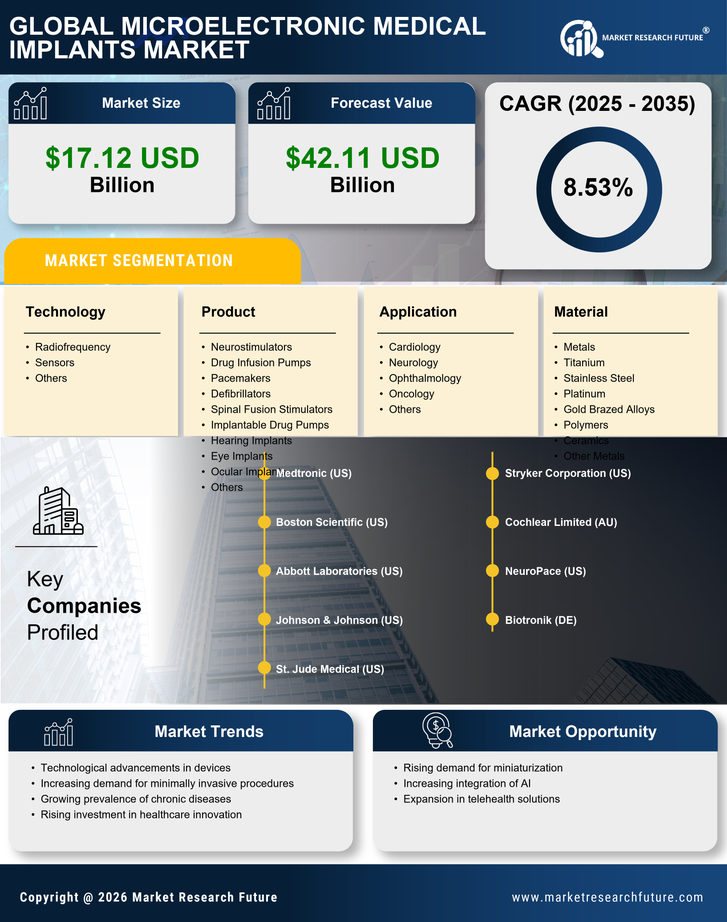
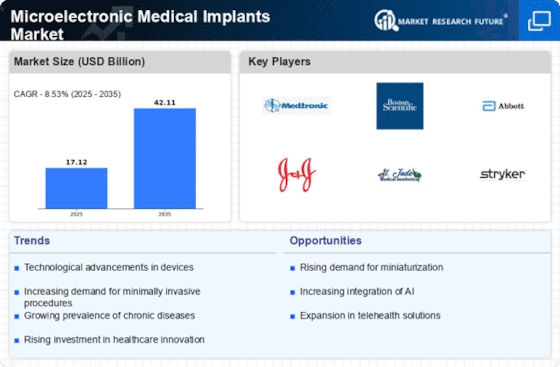
The increasing incidence of chronic diseases such as diabetes, cardiovascular disorders, and neurological conditions is a primary driver for the Microelectronic Medical Implants Market. As these health issues become more prevalent, the demand for advanced medical solutions, including microelectronic implants, is likely to rise. According to recent data, chronic diseases account for approximately 70% of all deaths worldwide, underscoring the urgent need for innovative treatment options. This trend suggests that healthcare providers are increasingly turning to microelectronic implants to enhance patient outcomes and improve quality of life. The Microelectronic Medical Implants Market is thus positioned to experience substantial growth as it addresses the needs of a growing patient population requiring long-term management of chronic conditions.
Technological Innovations in Implant Design
Technological advancements in the design and functionality of microelectronic medical implants are significantly influencing the Microelectronic Medical Implants Market. Innovations such as miniaturization, biocompatibility, and enhanced battery life are making implants more effective and user-friendly. For instance, the development of smart implants that can monitor health metrics in real-time is gaining traction. This evolution not only improves patient compliance but also allows for better data collection and analysis, which can lead to improved treatment protocols. The market is projected to grow as these technological innovations continue to emerge, potentially leading to a market valuation exceeding several billion dollars in the coming years.
Growing Demand for Minimally Invasive Procedures
The shift towards minimally invasive surgical techniques is a notable driver for the Microelectronic Medical Implants Market. Patients increasingly prefer procedures that promise reduced recovery times, less pain, and minimal scarring. Microelectronic implants, which can often be inserted with smaller incisions, align well with this trend. Data indicates that minimally invasive surgeries are expected to grow at a compound annual growth rate of over 10% in the next few years. This growth is likely to propel the demand for microelectronic implants, as they are integral to many of these advanced surgical techniques. Consequently, the Microelectronic Medical Implants Market stands to benefit from this evolving surgical landscape.
Increased Investment in Healthcare Infrastructure
Investment in healthcare infrastructure is a critical driver for the Microelectronic Medical Implants Market. Governments and private entities are allocating substantial resources to enhance healthcare facilities and technologies, particularly in emerging markets. This investment is likely to facilitate the adoption of advanced medical technologies, including microelectronic implants. For instance, initiatives aimed at improving access to healthcare services in underserved regions are expected to boost the demand for innovative medical solutions. As healthcare systems evolve and expand, the Microelectronic Medical Implants Market is poised to capitalize on these developments, potentially leading to increased market penetration and growth.
Rising Awareness and Acceptance of Implant Technologies
The growing awareness and acceptance of microelectronic implant technologies among patients and healthcare providers is a significant driver for the Microelectronic Medical Implants Market. Educational campaigns and successful case studies are helping to demystify these technologies, leading to greater patient confidence in their use. As more individuals become informed about the benefits of microelectronic implants, including improved health outcomes and enhanced quality of life, the demand is likely to increase. Furthermore, healthcare professionals are increasingly recognizing the advantages of these implants in treatment plans, which could further stimulate market growth. The Microelectronic Medical Implants Market is thus expected to thrive as acceptance continues to rise.